CHAPTER -4 “STRUCTURE OF ATOM” CONCEPTDETAILS
KEY CONCEPTS : [ *rating as per the significance of concept]
| 1. Dalton’s Atomic theory | ** |
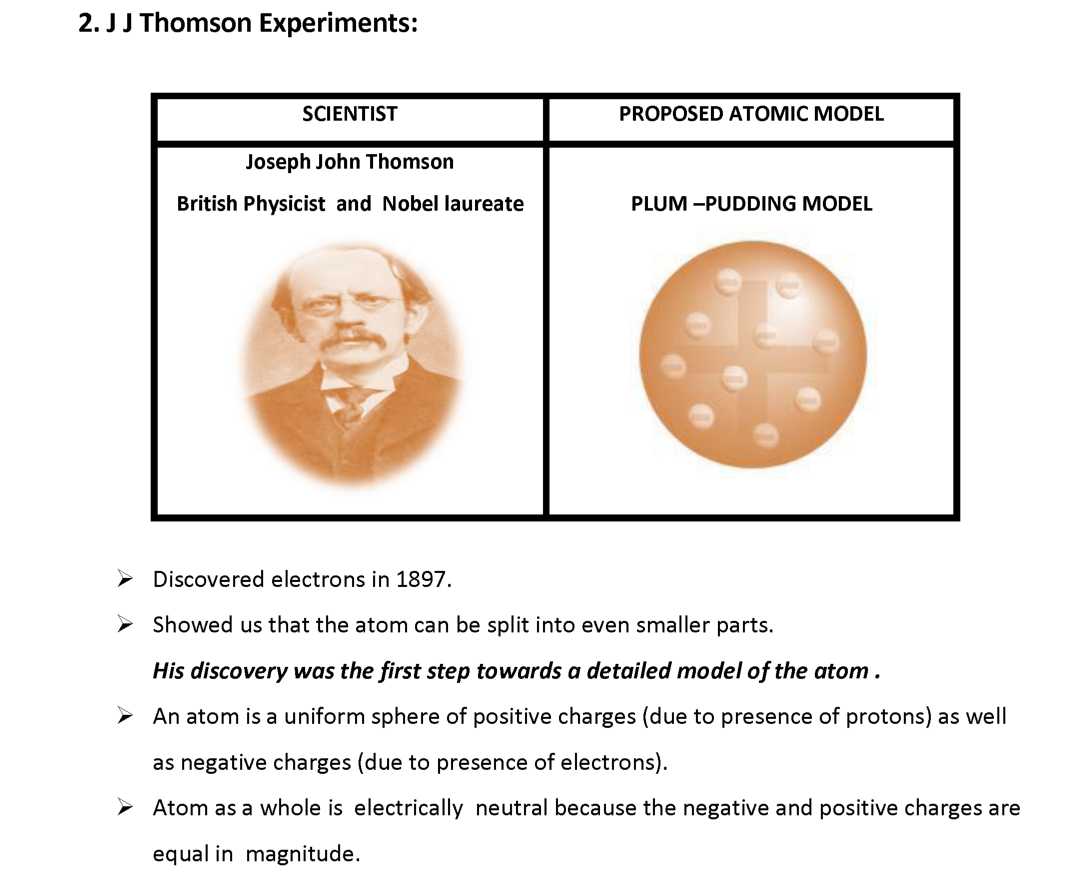
| 2. J J Thomson Experiments | *** |
| 3. Rutherford’s Scattering Experiments | ******** |
| 4. Sub atomic particles | ****** |
| 5. Atomic number & Mass number | ***** |
| 6. Neil Bohr’s Atomic Model | *** |
| 7. Electronic Configuration & Valency | ******* |
| 8. Isotopes & Isobars | **** |
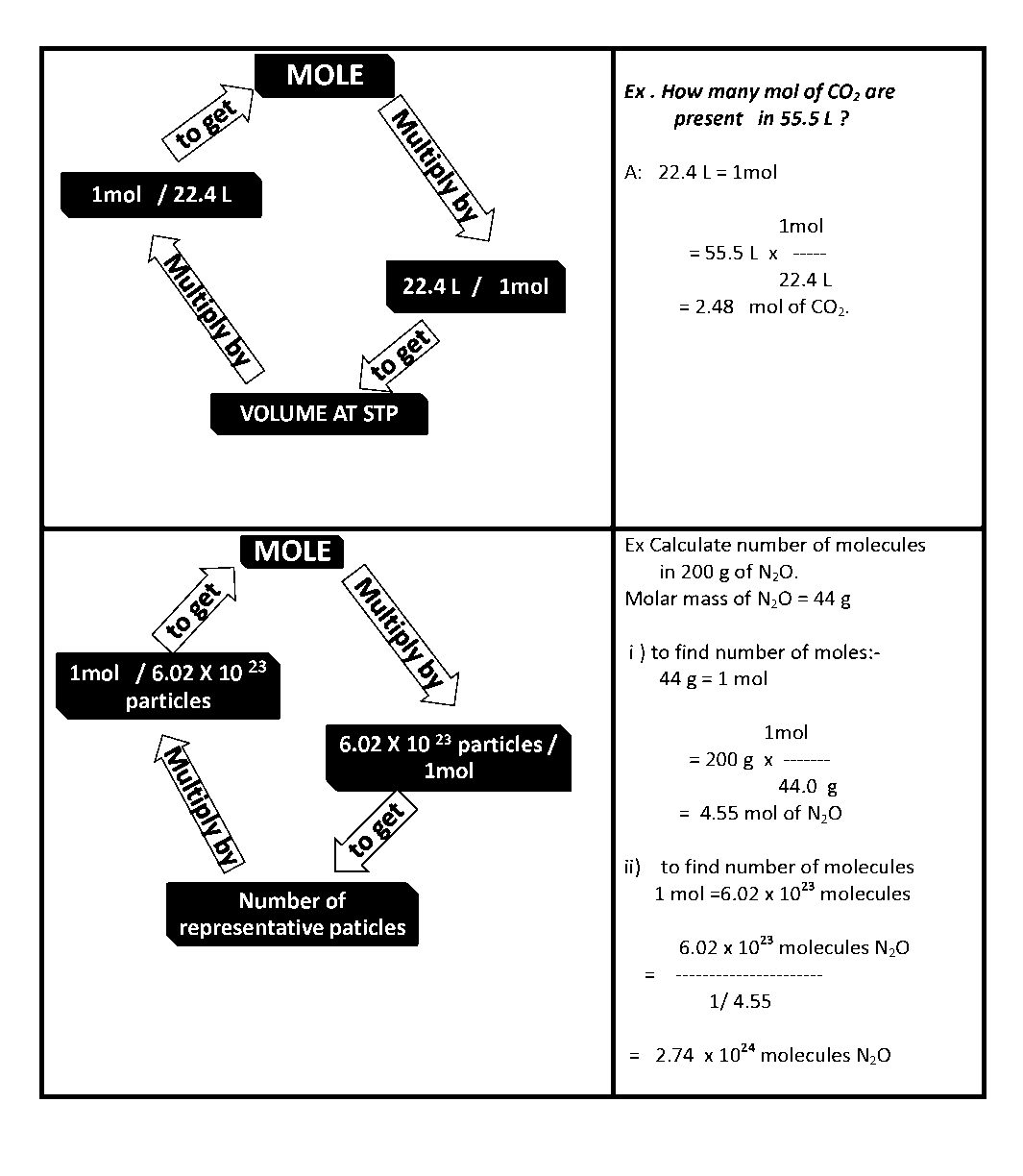
Pre requisites:- Difference between an atom & molecule.
- Gram atomic mass & Molar mass.
- Dalton’s Atomic theory.
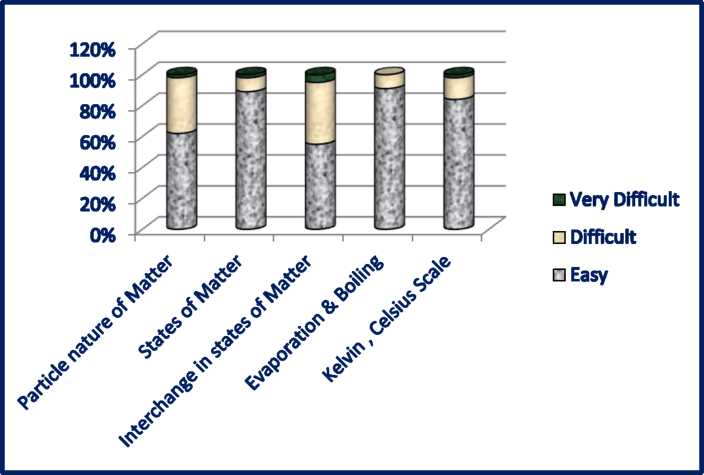
SURVEY ANALYSIS
Conceptual levels of comprehension on the basis of feedback taken from the students


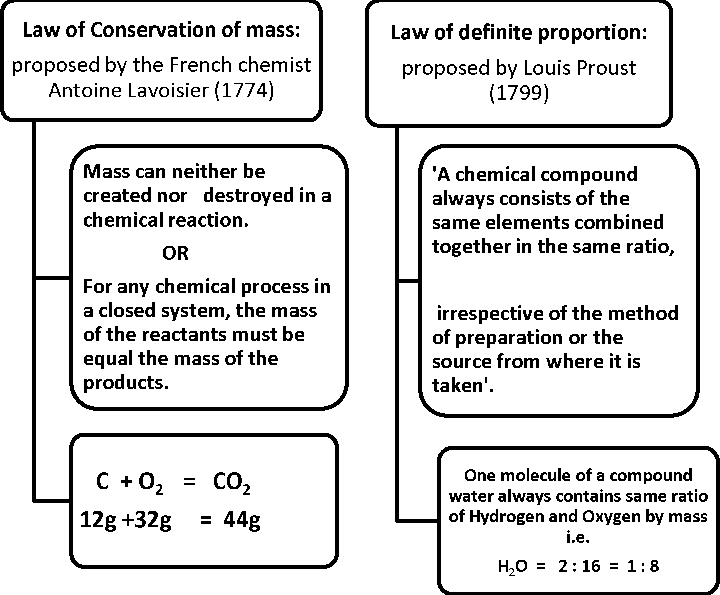
- First recorded evidence that atoms existed.
- Using his theory, Dalton rationalized the various laws of chemical combination
Dalton’s theory was based on the premise that the atoms of different elements could be distinguished by differences in their weights.
- Limitations
o The indivisibility of an atom was proved wrong , for, an atom can be further subdivided into protons, neutrons and electrons. o The atoms of same element are similar in all respects , but isotopes of same element have different mass.
Dalton’s theory was based on the premise that the atoms of different elements could be distinguished by differences in their weights.
- An electron is a negatively charged component of an atom which exists outside the nucleus. Each electron carries one unit of negative charge and has a very small mass as compared with that of a neutron or proton.
 Effects of Electric Field on Cathode Rays
Effects of Electric Field on Cathode Rays
JJ Thomson used cathode ray tubes to demonstrate that the cathode ray responds to both magnetic and electric fields.
Since the ray was attracted to a positive electric plate placed over the cathode ray tube (beam deflected toward the positive plate) he determined that the ray must be composed of negatively charged particles.
He called these negative particles “electrons.”
Limitation: Model failed to explain how protons and electrons were arranged in atom so close to each other.

- E. Goldstein in 1886 discovered the presence of new radiations in a gas discharge and called them canal rays. These rays were positively charged radiations which ultimately led to the discovery of another sub-atomic particle.
- Used a Cathode Ray Tube to study “canal rays” which had electrical and magnetic properties opposite of an electron
- Canal Rays: The positively charged radiation produced in the discharge tube at low pressure and high voltage are called canal rays.
Protons:
The canal rays have positively charged sub-atomic, particles known as protons (p).
Q.1 What was the model of an atom proposed by Thomson? Q.2 What is the nature of charge on electrons?
Q.3 What are canal rays ?
Q.4 State the nature of the constituents of canal rays.

Experiment: Rutherford took a thin gold foil and made alpha particles , [ He2+ ] positively charged Helium fall on it.
| S.No | OBSERVATION | INFERENCE |
| 1. | Most of the a-particles passed through | Most of the space inside the atom is |
| the gold foil without getting deflected. | empty. | |
| 2. | Very few particles were deflected. | Positive charge of the atom occupies |
| very little space. | ||
| 3. | A very few alpha particles, 1 in 100000 | Nucleus of an atom is very small as |
| completely rebound on hitting the gold | compared to the total size. | |
| foil. |

- Limitation: In Rutherford’s atomic model , Nucleus & electrons are held together by electrostatic force of attraction which would lead to the fusion between them. This does not happen in the atom.
Atomic radius ~ 100 pm = 1 x 10-10 m Nuclear radius ~ 5 x 10-3 pm = 5 x 10-15 m

- In 1932, James Chadwick proved that the atomic nucleus contained a neutral particle which had been proposed more than a decade earlier by Ernest Rutherford officially discovered the neutron in 1932,
- Chadwick received the Nobel Prize in 1935.
A neutron is a subatomic particle contained in the atomic nucleus. It has no net electric charge, unlike the proton’s positive electric charge.
Q.1 Who discovered the nucleus of the atom?
Q.2 What is the charge on alpha particles ?
Q.3 Which observation of Rutherford’s scattering experiment established the presence large empty space in atom?
Q.4 What is the nature of charge on nucleus of atom?
Q.5 Who discovered neutron ?
| Name | Symbol | Location in the atom | Charge | Relative Mass | Actual Mass (g) |
| Electron | E | Around the nucleus | 1- | 1/1840 | 9.11 x 10 -28 |
| Proton | P | In the nucleus | 1+ | 1 | 1.67 x 10 -24 |
| Neutron | n | In the nucleus | 0 | 1 | 1.67 x 10 -24 |
Protons & Neutrons collectively are known as NUCLEONS.
Q.1 Why is the relative mass of an electron is taken as negligible ? Q.2 Give the actual masses of electron & proton in kg?
Q.3 What are nucleons?
“Atomic number of an element is defined as the number of unit positive charges on the nucleus (nuclear charge) of the atom of that element or as the number of protons present in the nucleus.”
Atomic number, Z = Number of unit positive charge on the nucleus = Total number of unit positive charges carried by all protons present in the nucleus.
= Number of protons in the nucleus (p)
= Number of electrons revolving in the orbits (e)
Eg :- Hydrogen – Atomic number = 1 (1 proton)
Helium – Atomic number = 2 (2 protons)
Mass number[ A] : It is defined as the sum of the number of protons & neutrons present in the nucleus of an atom.
Mass Number = Mass of protons + Mass of neutrons Eg :- Carbon – Mass number = 12 (6 protons + 6 neutrons) Mass = 12u Aluminium – Mass number = 27 (13 protons + 14 neutrons) Mass = 27u



 Main Postulates of the Bohr Model [refer NCERT Text Book article 4.3 page number-49]
Main Postulates of the Bohr Model [refer NCERT Text Book article 4.3 page number-49]
Q.1 What happens when an electron jumps from lower to higher energy level?
Q.2 Which energy shell is nearest to the nucleus of an atom?
Q.3 Which energy shell has higher energy L or N ?
- Electronic configuration & Valency: Bohr and Bury Scheme – Important Rules
| S.No | Electron Shell |
2n2
where n = shell number |
Maximum Capacity |
| 1 | K Shell | 2 x (1) 2 | 2 electrons |
| 2 | L Shell | 2 x (2) 2 | 8 electrons |
| 3 | M shell | 2 x (3) 2 | 18 electrons |
| 4 | N shell | 2 x (4) 2 | 32 electrons |
The outermost shell of an atom cannot accommodate more than 8 electrons, even if it has a capacity to accommodate more electrons. This is a very important rule and is also called the OCTET RULE. The presence of 8 electrons in the outermost shell makes the atom very stable.
Q.1 An atoms has atomic number 13. What would be its configuration.
Q.2 What is octet rule?
Q.3 How many electrons M shell can accommodate?
Q.4 If an atom has complete K and L shell, what would be its atomic number?
| ISOTOPES | ISOBARS |
| Chemically same , physically different | Chemically different , physically same |
| Number of electrons is same | Number of electrons is different . |
| Cannot be separated by chemical means | Can be separated by chemical means |
[ for application of isotopes refer NCERT Text Book article 4.6 page number-53]
Q.1 Why atoms of isotopes are chemically same?
Q.2 Give the representation of three isotopes of carbon which are C-12, C-13 and C-14.
- Mark Questions:
- Write the names of three elementary particles which constitute an atom.
- Name the scientist & his experiment to prove that nucleus of an atom is positively charged.
- Which is heavier , neutron or proton ?
- *How many times a proton is heavier than an electron?
- What was the model of an atom proposed by Thomson ?
- How many electrons at the maximum can be present in the first shell ?
- What type of charge is present on the nucleus of an atom?
- Give the number of protons in 35Cl17
- *What are iso bars ?
- Name the particles which determine the mass of an atom.
- Marks Questions:
- Define the following terms: a) Atomic number b) Mass number
- Write the charges on sub atomic particles.
- Identify the isotopes out of A , B , C & D ? 33A17 , 40B20 , 37C17 , 38D19
- * Give one Achievement and one limitation of J.J Thomson’s model of atom?
- What are valence electrons? Give example.
- *Which kind of elements have tendency to lose electron ? Give example.
- How many electrons are present in the valence shell of nitrogen & argon?
- State the maximum capacity of various shells to accommodate electrons.
- Give the symbol , relative charge & mass of the three sub atomic particles.
- From the symbol 32 S16 state :
i) Atomic number of sulphur, ii) Mass number of sulphur iii) Electronic configuration of sulphur.
- Marks Questions:
- Why do Helium has Zero valency?
- An atom contains 3 protons , 3 electrons and 4 neutrons .What is its atomic number , mass number & valency?
- *How are the isotopes of hydrogen represented ?
- Write the complete symbol for the atom with the given atomic number [Z] & mass
number[A].
i) Z= 17 , A = 15 ; ii) Z=4 , A = 9; iii) Z= 92 ; A=233
- *What would be the electronic configuration of Na+ , Al3+ , O2- , Cl –.
- * a) Give the observations as well as inferences of Rutherford’s Scattering experiment for determining the structure of an atom.
b) On the basis of above experiment write the main features of atomic model.
- Write the main postulates of Bohr’s Model of Atom.
- The scientists who discovered subatomic particles.
- Rutherford established the existence of nucleus.
- Difference between Atomic number and Mass number
- Electronic configuration & its relation with Valency.
- Difference between Isotope and Isobar.