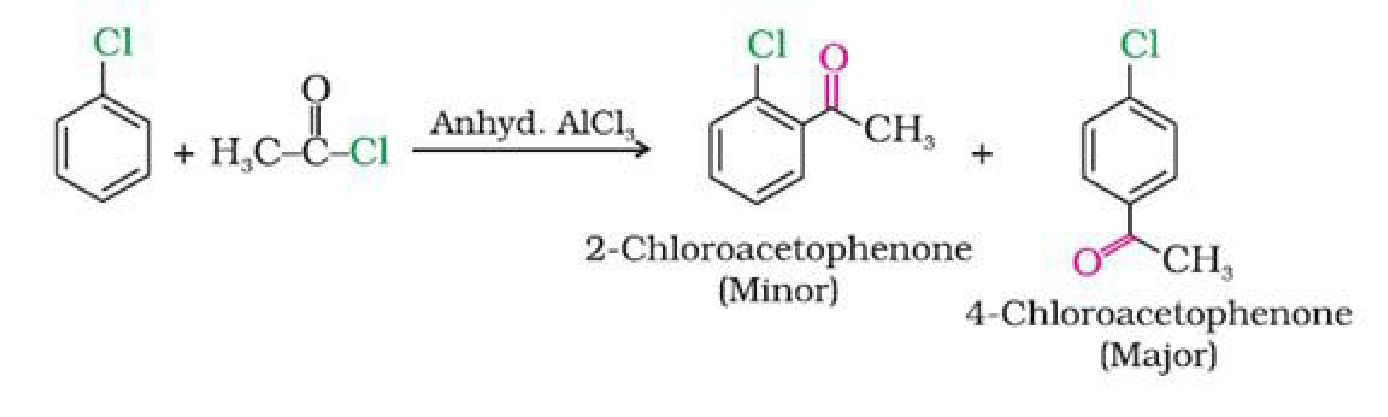
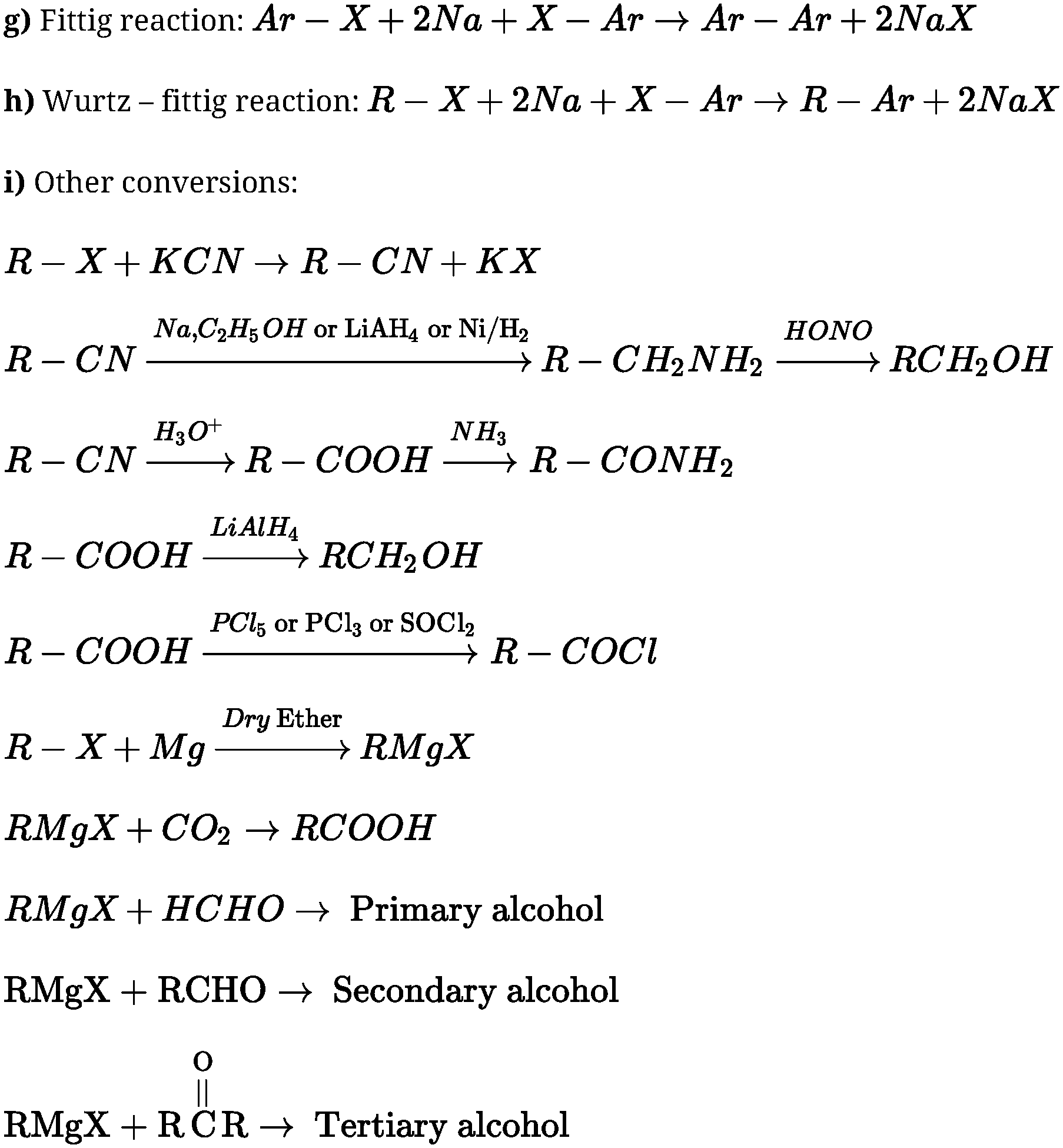गिल्लू पाठ का सार


Hindi Sanchayan Summary Notes for Class 9th of all Chapters
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 16
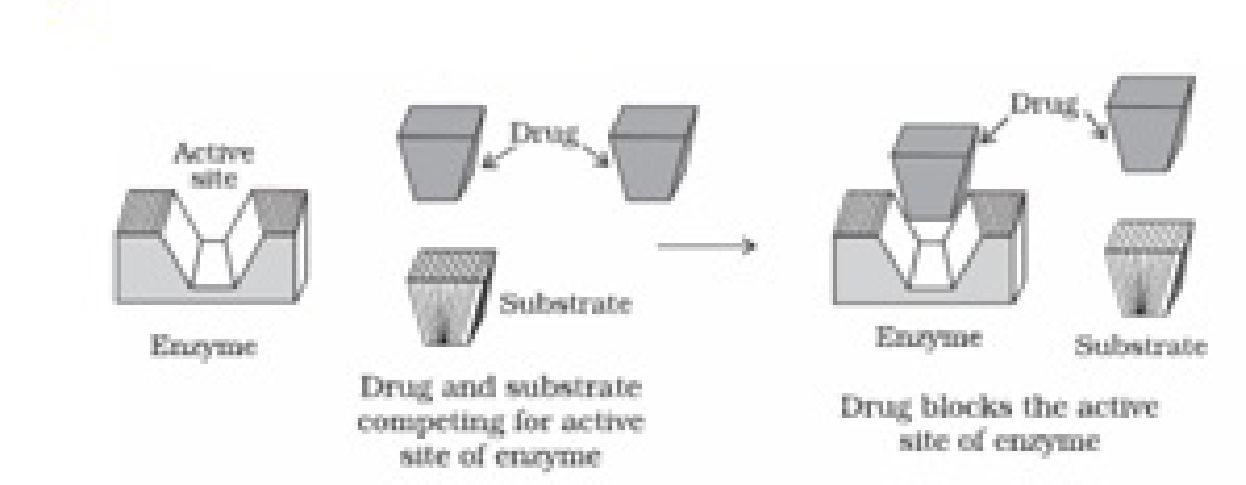
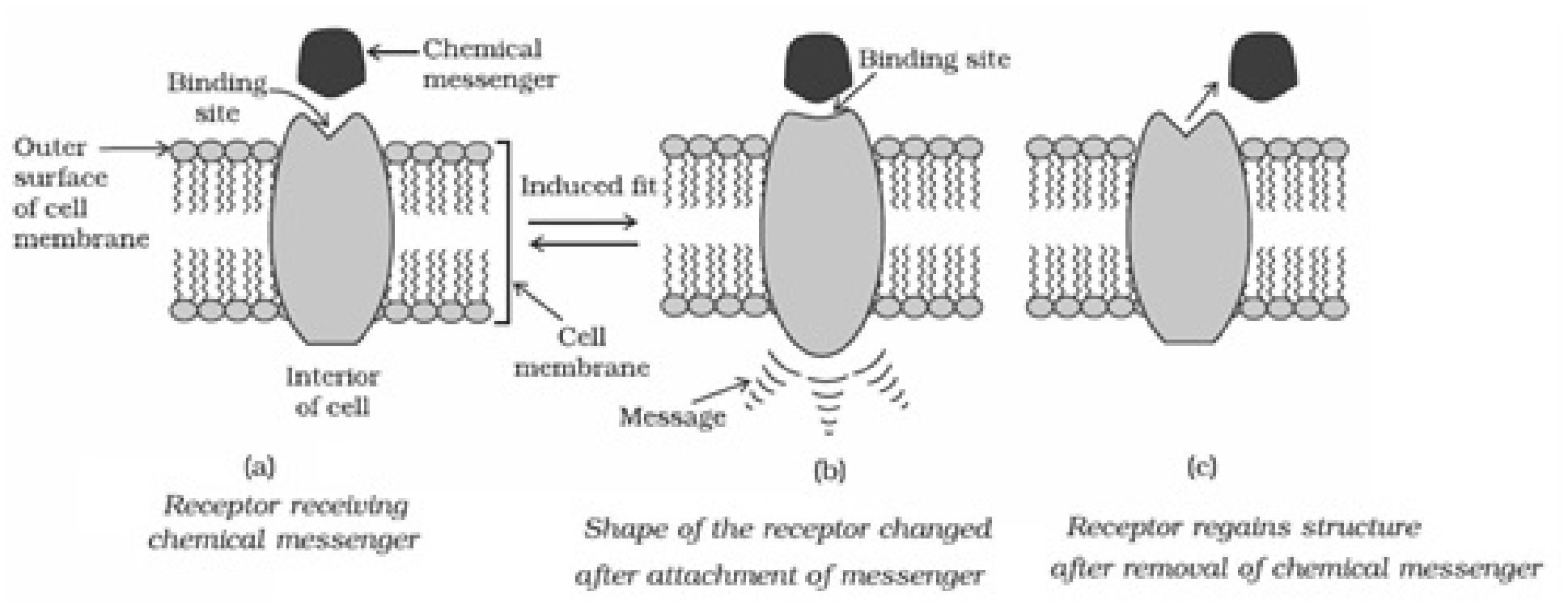
natural substrate for their attachment on the active sites of enzymes.
Ar I 1 ■ ■ r■ blli> ur| I fl
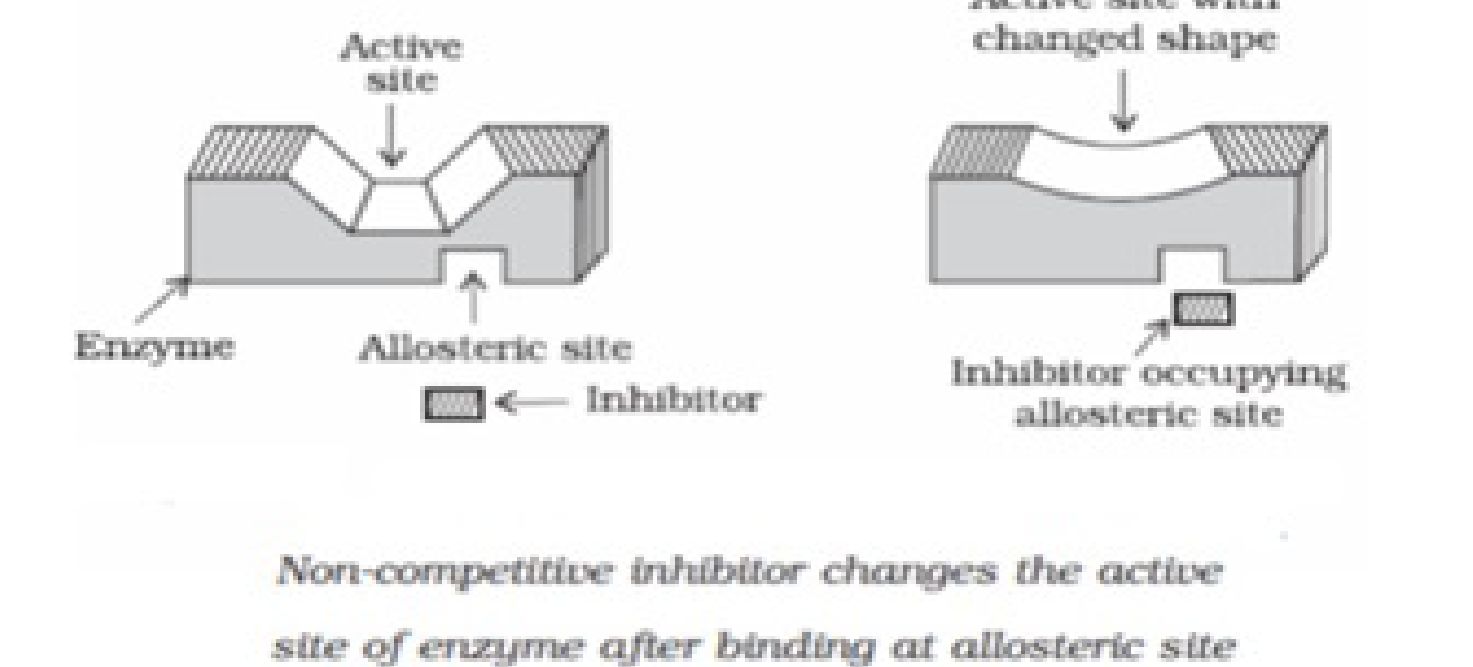
Classification of antimicrobial drugs based on the mode of control of microbial
diseases:
Classification of antimicrobial drugs based on its spectrum of action:
CH2^H
!
o
CH2– O-C-C17H3s
I O
M
CH – O – C – C17Hjs + 3NaOH
I O
J A [1 * .,
CH2– O-C-C17Hjs
Glyceryl ester Sodium
of stearic acid (Fat) hydroxide
» 3CiyHJSCOONa + CH -OH
I
(Soap) CH1^H
Sodium Gtycerol
stearate
This reaction is known as saponification.
scraping off the soaps in small broken pieces.
2C17HyCOONa-CaCl- ^ % NaCl– Ca
^oap ftGoltfbte Cd L. Lur.
::=:^i:r (ioap)
2C-H3jCOOXa+MgCl3 ^2 NaCl-(C-H3jCOO)3Mg
iQap ir.E■:■ It’Dh ™ zr.dE i.um
E-[ddfd te (EC-Erp)
These precipitates stick to the fibres of the cloth as gummy mass and block the ability of
soaps to remove oil and grease from fabrics. Therefore, it interferes with the cleansing
ability of the soap and makes the cleansing process difficult.
In acidic medium, the acid present in solution precipitate the insoluble free fatty acids which
adhere to the fabrics and hence block the ability of soaps to remove oil and grease from the
fabrics. Hence soaps cannot be used in acidic medium
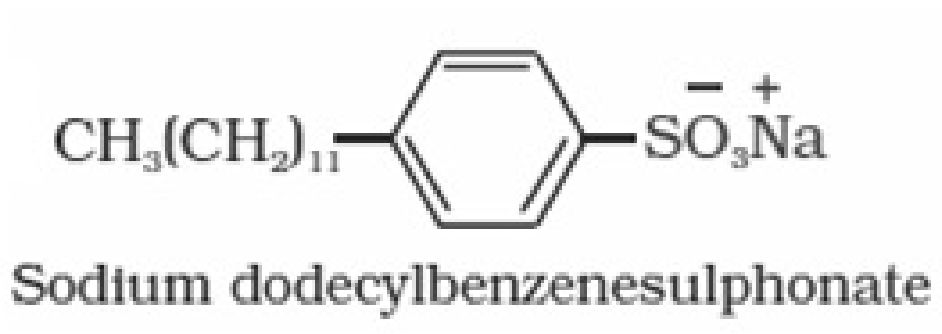
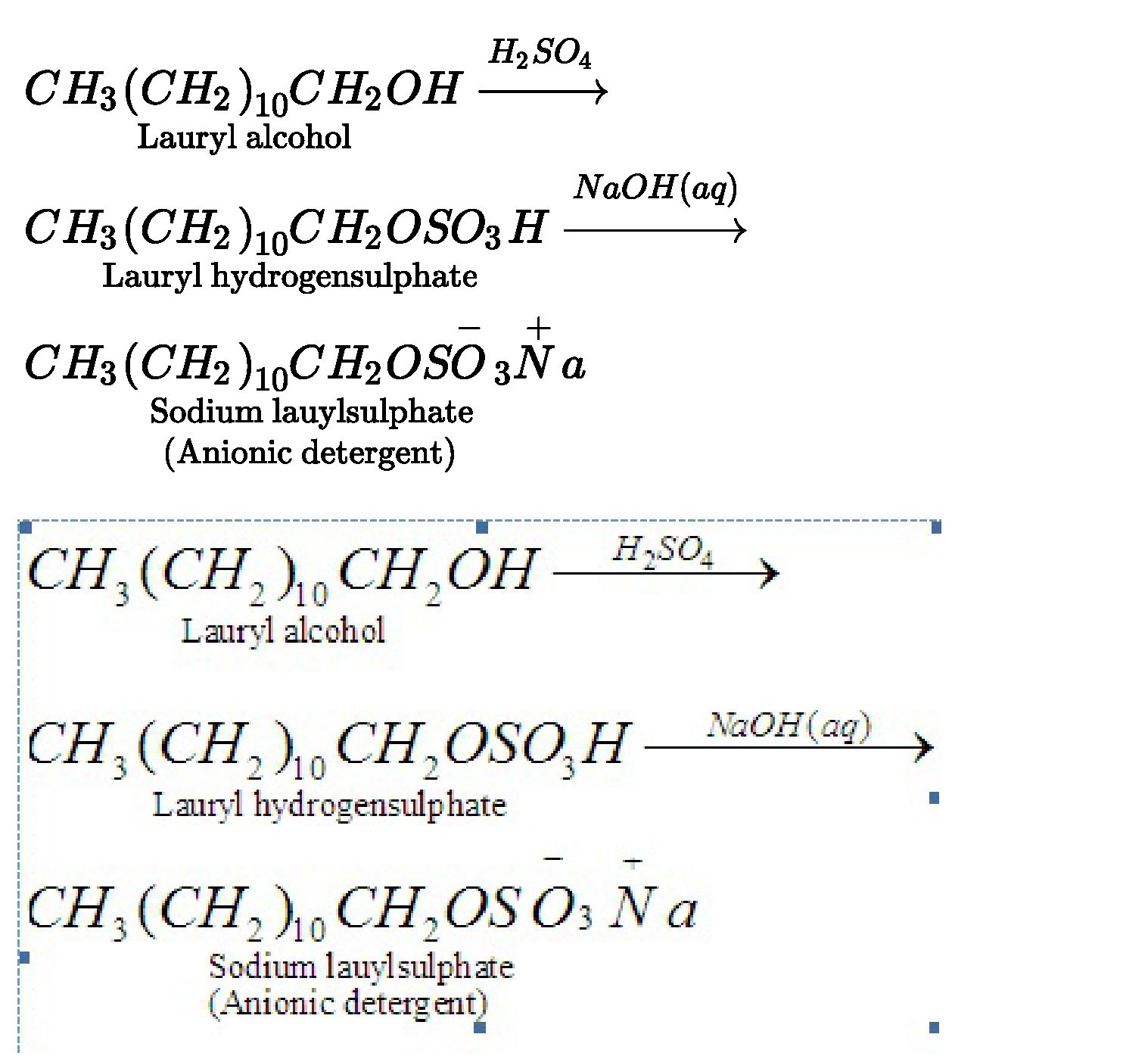
• Detergents: Detergents are sodium salts of long chain of alkyl benzene sulphonic
acids or sodium salts of long chain of alkyl hydrogen sulphates.
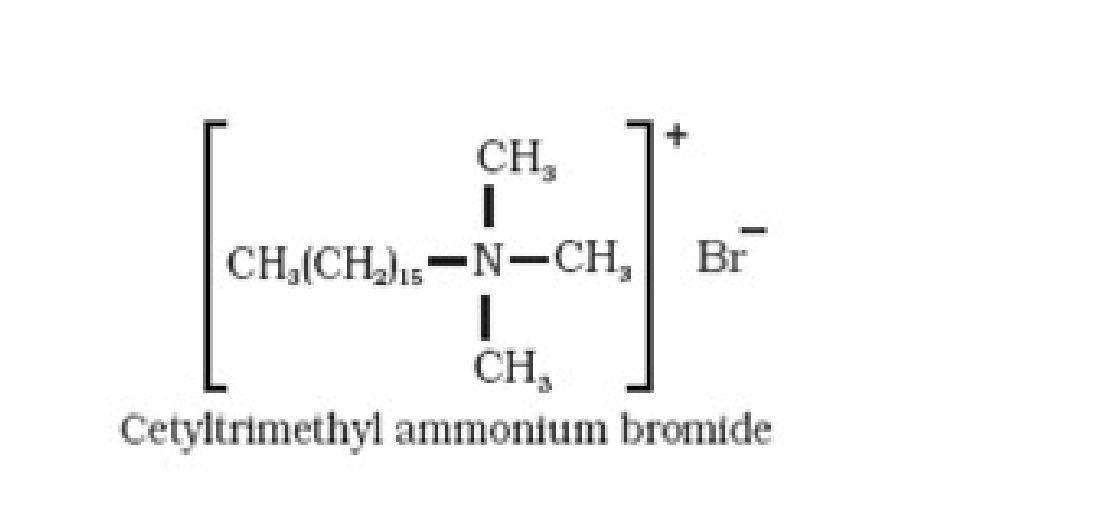
• Classification of detergents:
They are used in household cleaning like dishwasher liquids, laundry liquid detergents,
laundry powdered detergents etc. They are effective in slightly acidic solutions where soaps
do not work efficiently.
Example: Detergent formed by condensation reaction between stearic acid reacts and poly
ethyl eneglycol.
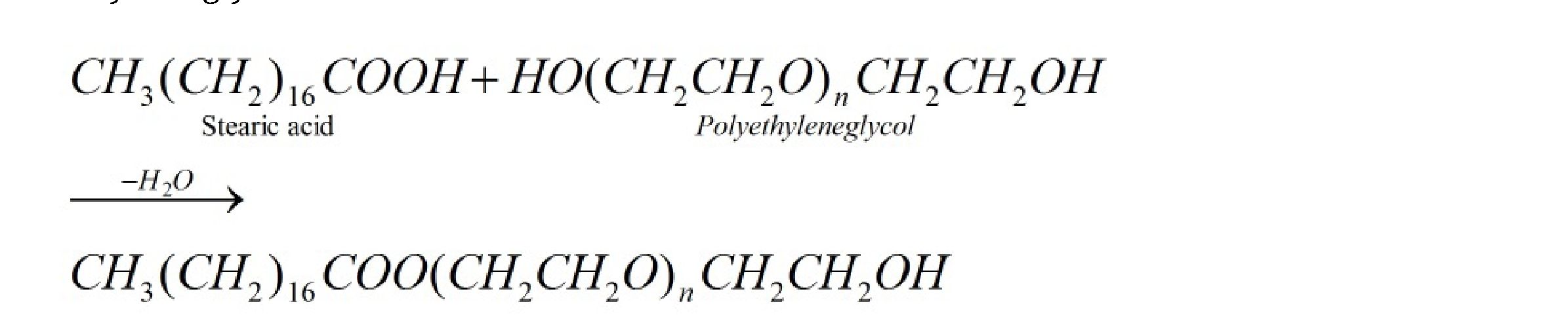
It is used in Making liquid washing detergents. They have effective H- bonding groups at one
end of the alkyl chain which make them freely water soluble.

CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 15
Polymers
Homopolymers:Polymers formed by the polymerisation of a single monomeric species.
Examples – Polythene, Polystyrene.
Copolymers:Polymers formed by addition polymerisation of two different monomers.
Examples – Buna-S, Buna -N.
Based on Molecular forces, it is classified into
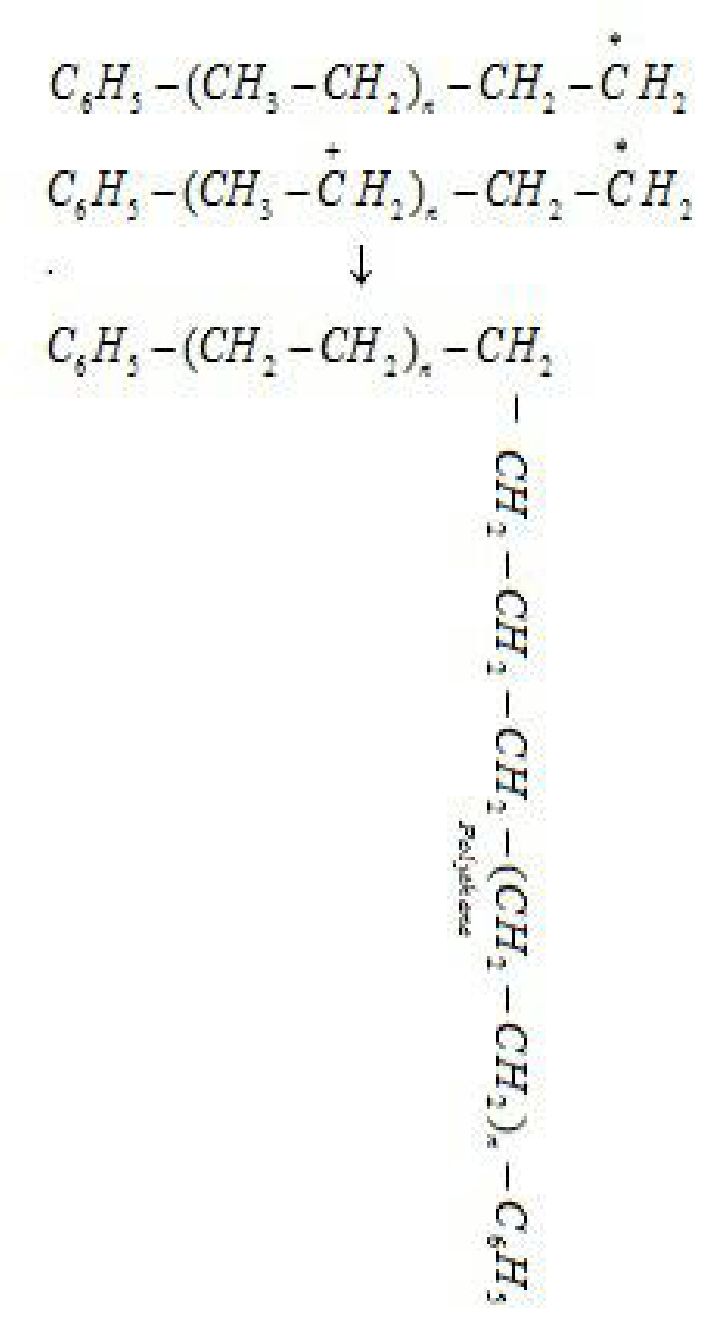
Step 1: Chain initiating step: Organic peroxides undergo homolytic fission to form free
radicals which acts as initiator. Initiator adds to C-C double bond of an alkene molecule to
form a new free radical
O O O
Ii fus I il . *
<VJ,^KW>C-Crt >2C^>0^-2CA +2C0,
Bcnznyi pmaKk ETwmi redjcaJ
* ■
C*H*+CHj=CKt * CJi,-CJi:-CHL
Step 2: Chain propagating step: Free radicals formed by homolytic cleavage adds to a double
bond of monomer to form a larger free radical. Radical formed adds to another alkene
molecule to form a larger free radical. This process continues until the radical is destroyed.
These steps are called propagation steps.
CiHi ~ CH1 – C H1 – CH1 = CH1
CJii – CH1 – CH1 – CH1 – C H1
¥
CiHi – CCH1 – CH^ – CH1 – C H1
Step 3: Chain terminating step: For termination of the long chain, free radicals combine in
different ways to form polythene. One mode of termination of chain is shown as under:
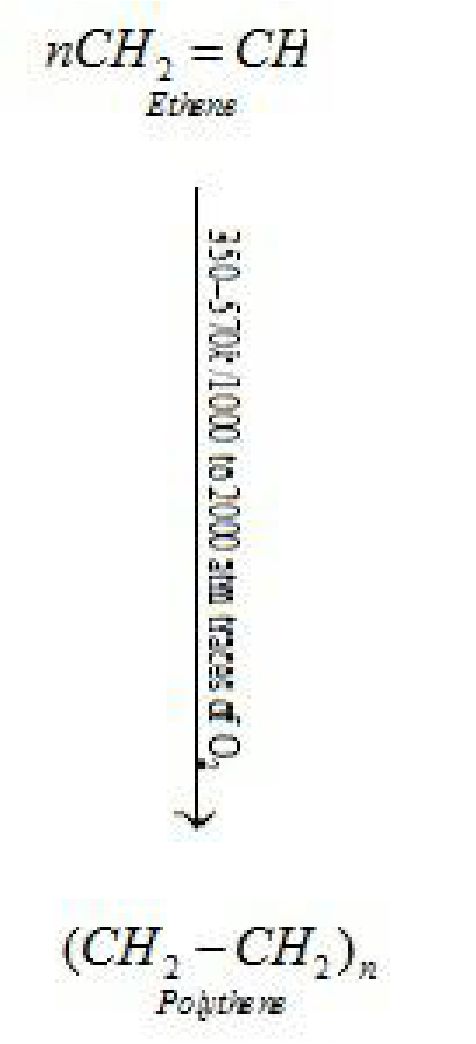
It is used in the insulation of electricity carrying wires and manufacture of squeeze bottles,
toys and flexible pipes

It is used for manufacturing buckets, dustbins, bottles, pipes, etc.
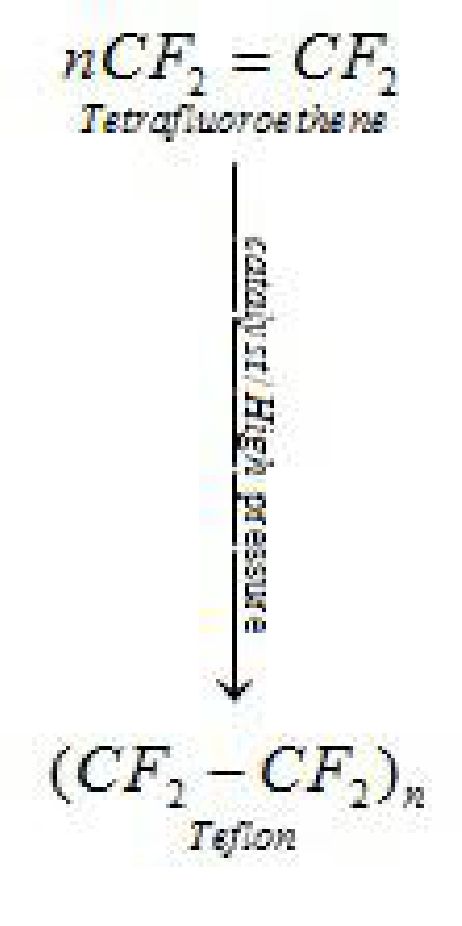
It is used in making oil seals and gaskets and also used for non – stick surface coated utensils
It is used as a substitute for wool in making commercial fibres such as orlon or acrilan.
1. Polyamides: Polymers possess amide linkage (-CONH-) in chain. Thesepolymers are
popularly known as nylons. Examples:
(a) Nylon 6, 6: It is prepared by the condensation polymerisation of hexamethylenediamine
with adipic acid under high pressure and at high temperature.
KHOOC(CH2)4COOH+ }TH2H(CH:)^XH2
It is used in making sheets, bristles for brushes and in textile industry.


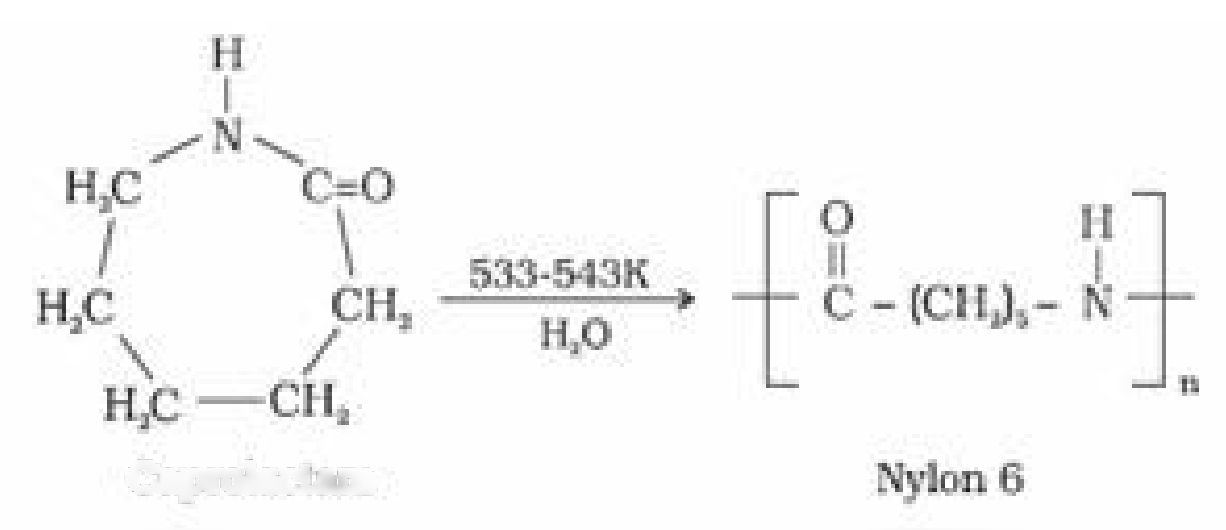
(b) Nylon 6: It is obtained by heating caprolactum with water at a high temperature
CupraUcLam
It is used for the manufacture of tyre cords, fabrics and ropes.
n HOHt- CH1OH t n HOOCn0- COOH —*
Efl^DK^ol TerepbLhabc ^rid
|Etha^!.2’dKfl (BmrKie-!.4 – dl
It is used to create resistance in polymerised product and is used in blending with cotton and
wool fibres and also as glass reinforcing materials in safety helmets, etc.
a). Bakelite: These are obtained by the condensation reaction of phenol with formaldehyde
in the presence of either an acid or a base catalyst. The initial product could be a linear
product – Novolac used in paints.

b). Novolac on heating with formaldehyde forms Bakelite
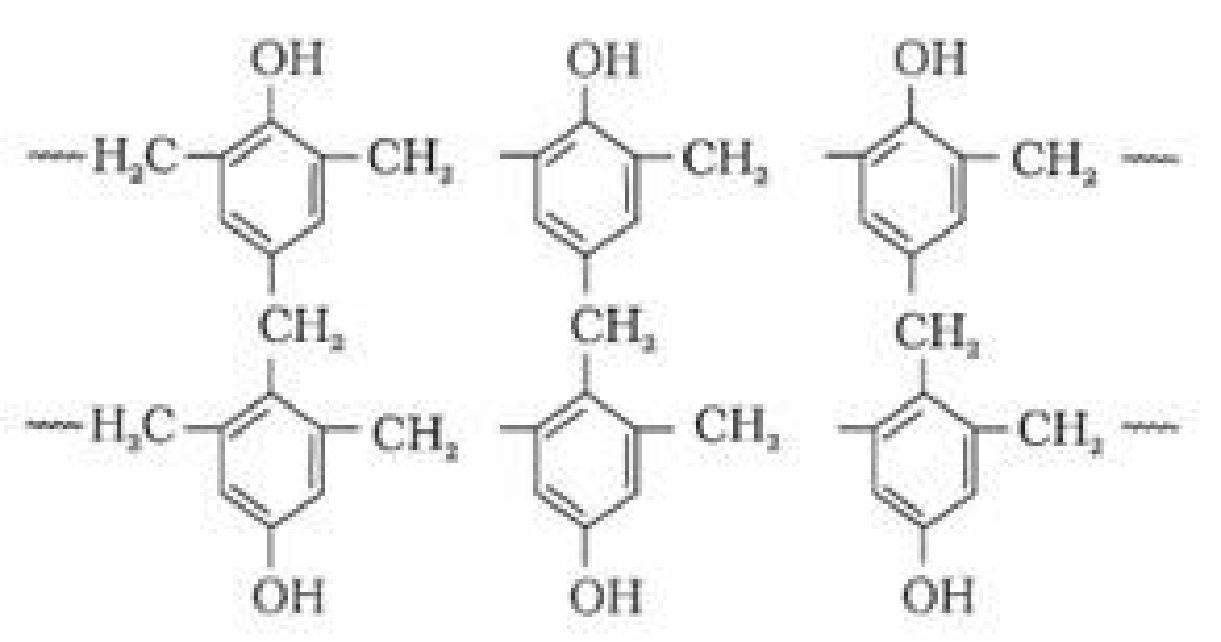
It is used for making combs, phonograph records, electrical switches and handles of various
utensils

It is used in the manufacture of unbreakable crockery.

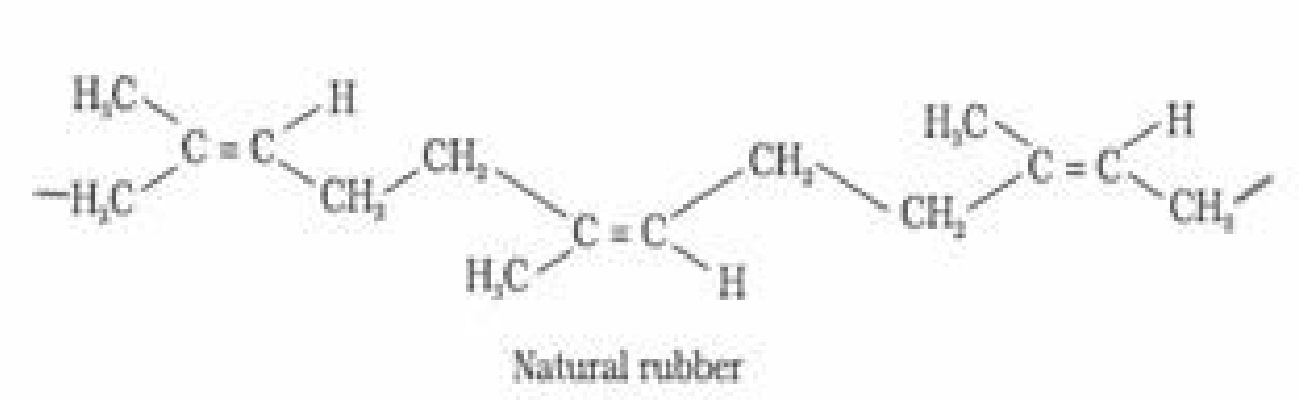
a). Natural rubber: Natural rubber is a linear polymer of isoprene (2-methyl-1, 3-butadiene)
and is also called as cis – 1, 4 – polyisoprene.
b). Synthetic rubber: Synthetic rubbers are either homopolymers of 1, 3 – butadiene
derivatives or copolymers of 1, 3 – butadiene or its derivatives with another unsaturated
monomer.

It is used for manufacturing conveyor belts, gaskets and hoses
B) Buna – N

It is used in making oil seals, tank lining, etc. because it is resistant to the action of petrol,
lubricating oil and organic solvents
C) Buna – S



It is used in speciality packaging, orthopaedic devices and in controlled release of drugs.
b). Nylon 2-nylon 6: It is an alternating polyamide copolymer of glycine(H2N-CH2-COOH)
and amino caproic acid (H2N (CH2)5 COOH)
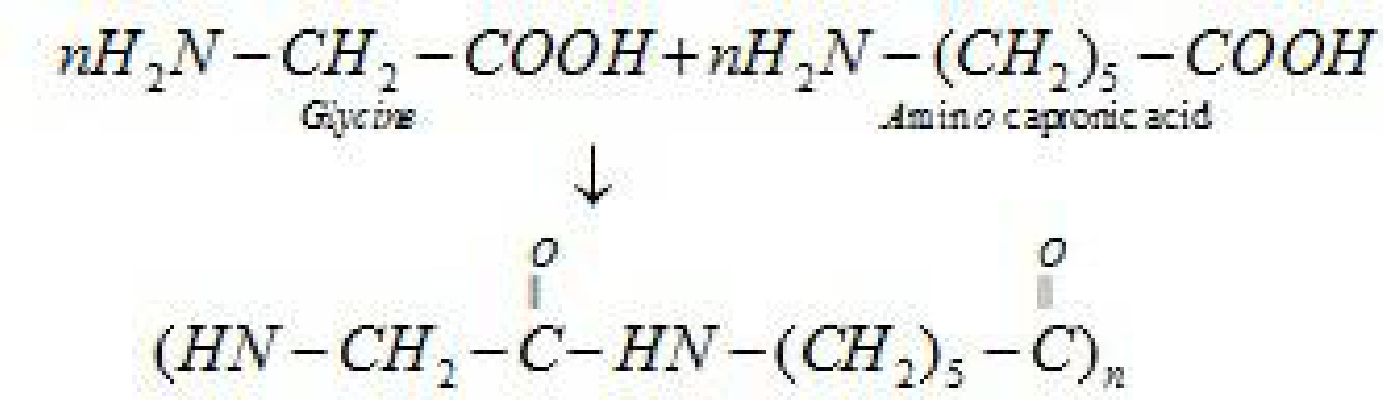
which on heating undergo extensive cross linking in moulds and eventually undergo a
permanent change. Examples – Bakelite, Urea-formaldelyde resins
Condensation Polymerisation or Step Growth polymerization: Polymerisation generally
involves a repetitive condensation reaction between two bi-functional monomers. In
condensation reactions, the product of each step is again a bi-functional species and the
sequence of condensation goes on. Since, each step produces a distinct functionalized species
and is independent of each other, this process is also called as step growth polymerisation.
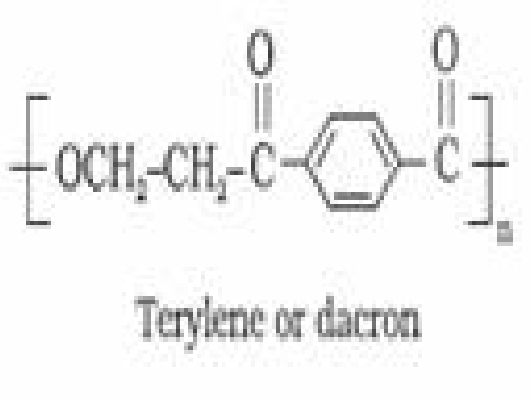
Terylene or Dacron: It is manufactured by heating a mixture of ethylene glycol and
terephthalic acid at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst.
Vulcanisation of rubber: The process of heating a mixture of raw rubber with sulphur and
an appropriate additive in a temperature range between 373 K to 415 K to improve upon
physical properties like elasticity, strength etc.
Biodegradable Polymers: Polymers which are degraded by microorganisms within a suitable
period so that biodegradable polymers and their degraded products do not cause any serious
effects on environment.
Examples of biodegradable polymer:
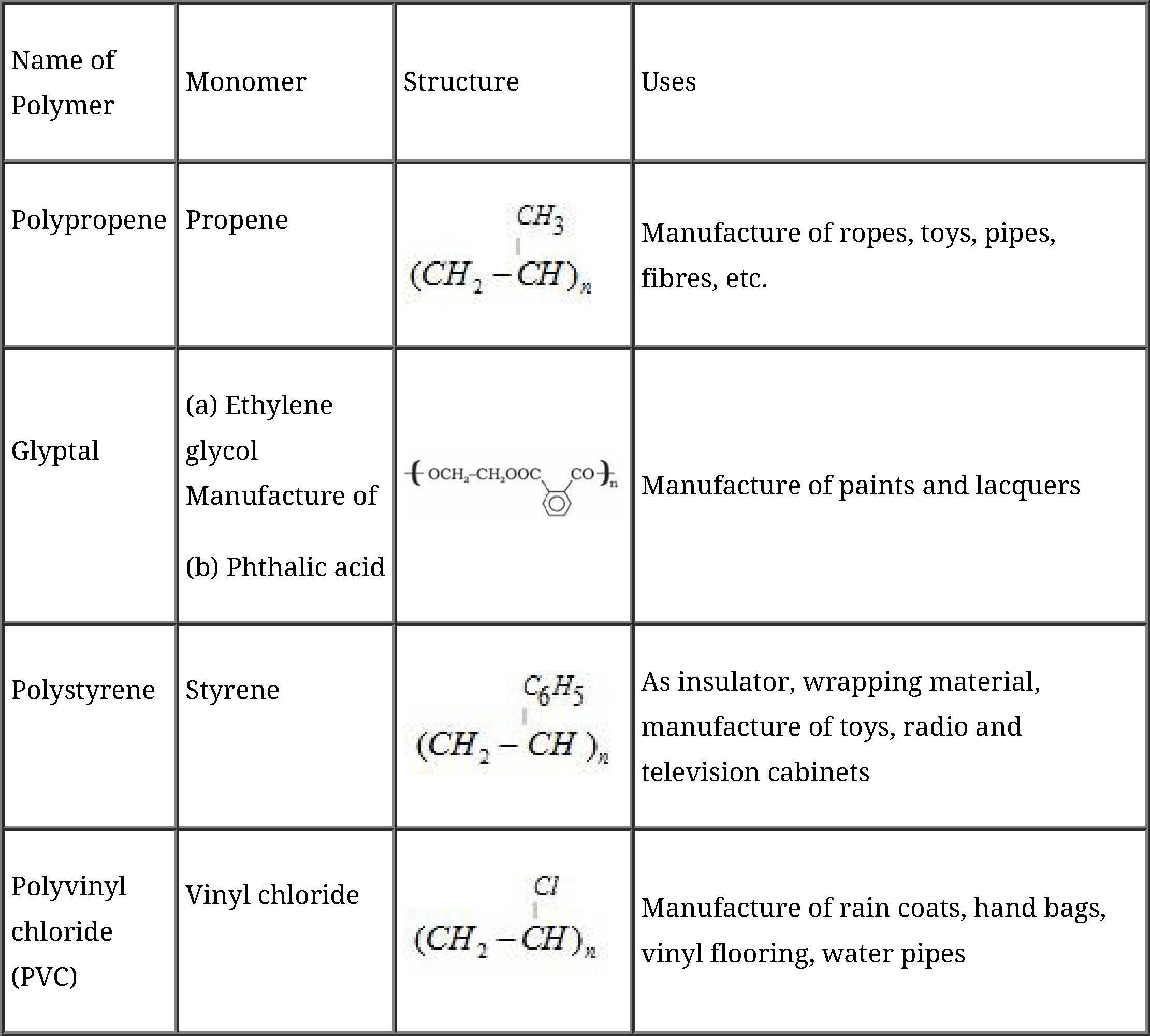
Commercially important polymers along with their structures and uses:
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 14
Biomolecules
Monosaccharides
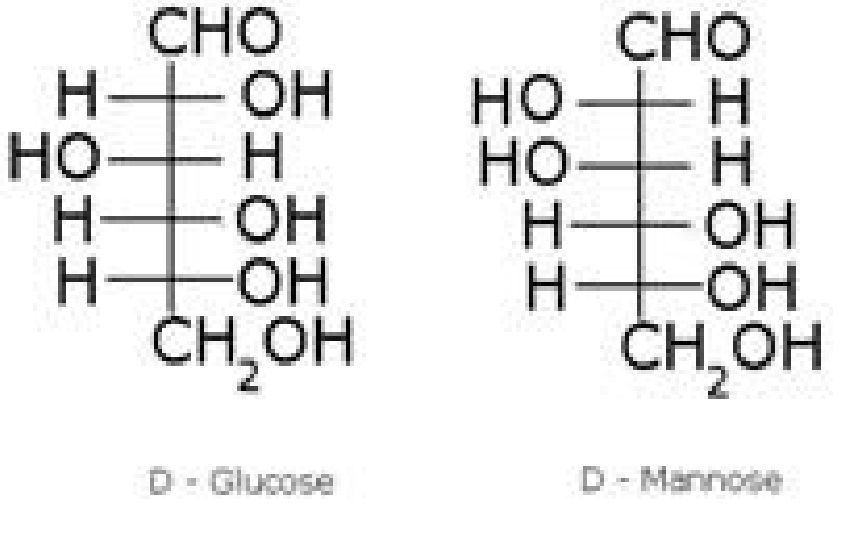
Preparation of glucose (also called dextrose, grape sugar):
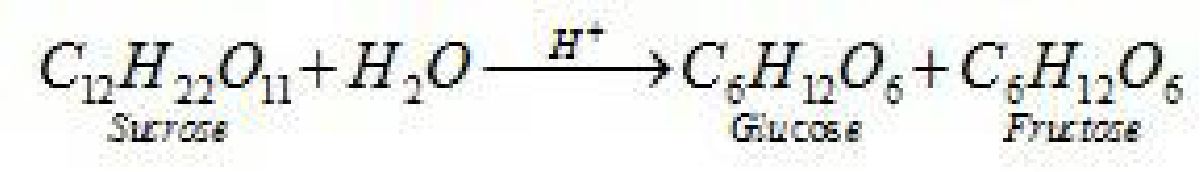
• From starch

• Structure of glucose
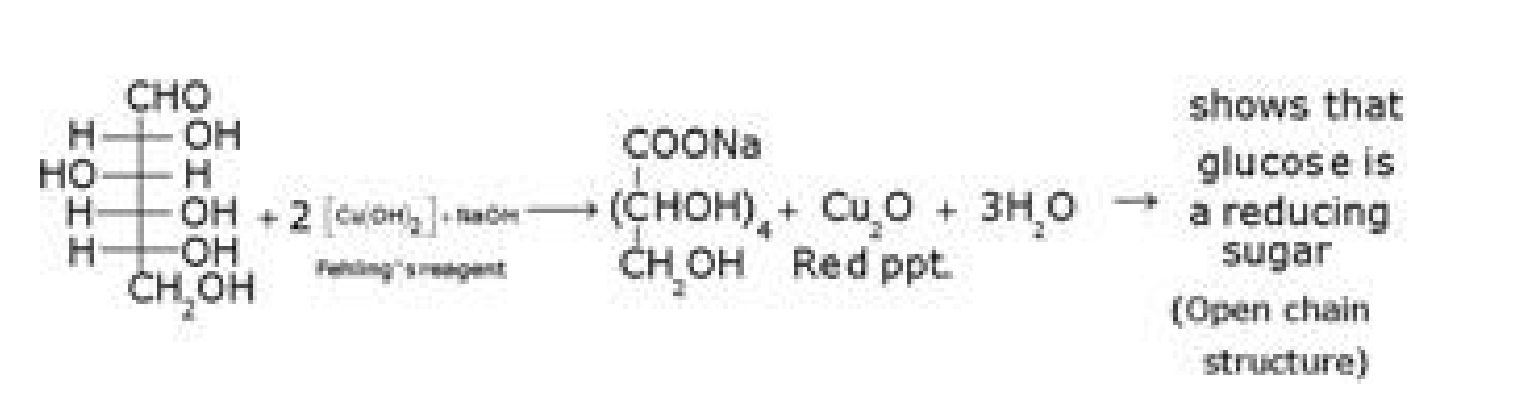
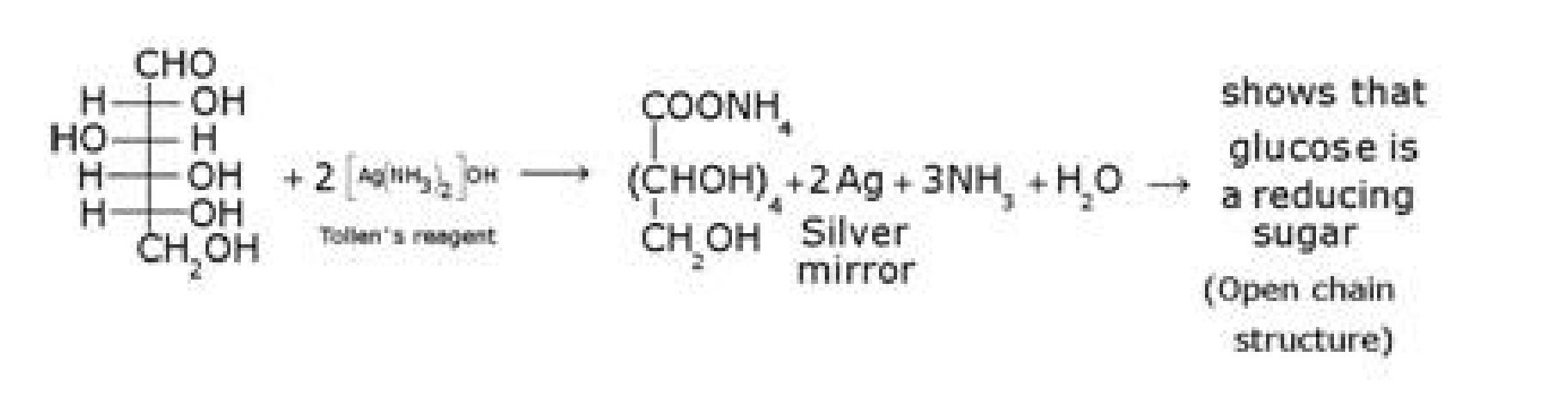
• Structure elucidation of glucose:
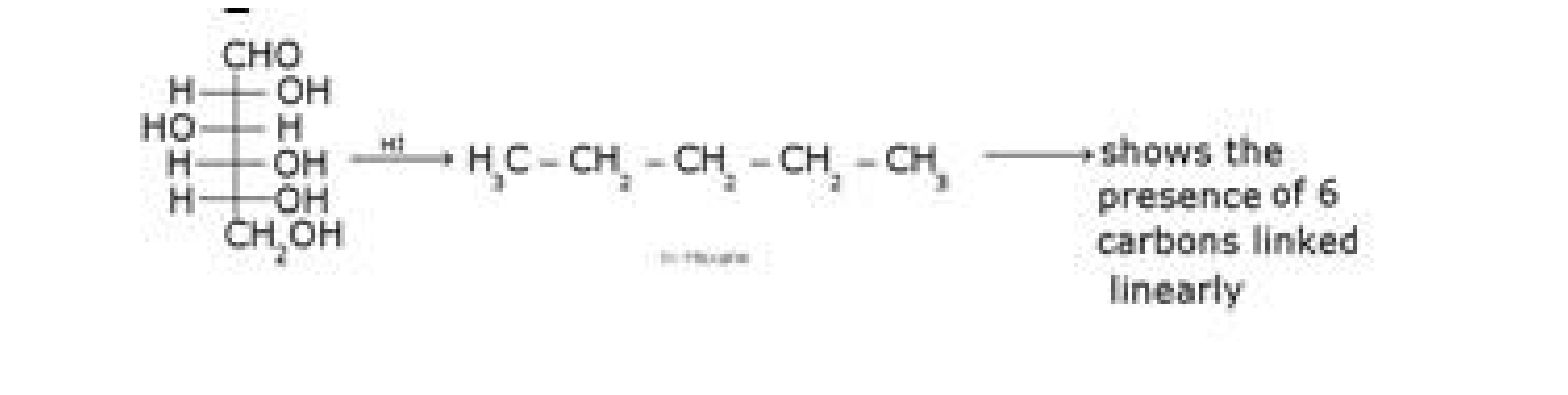
b) D – glucose with HCN
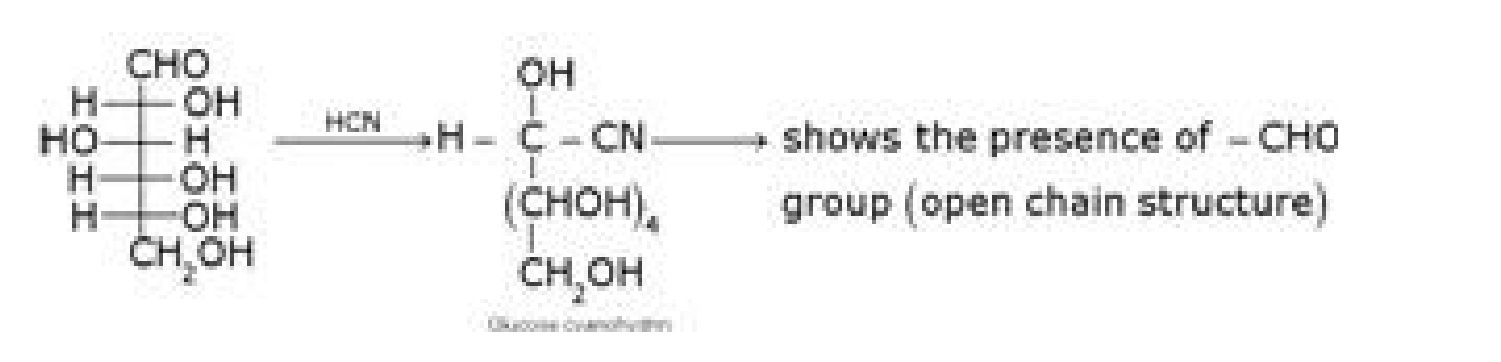
c) D – glucose with NH2OH

d) D- glucose with Fehling’s reagent
/ 15
e) D – glucose with Tollen’s reagent
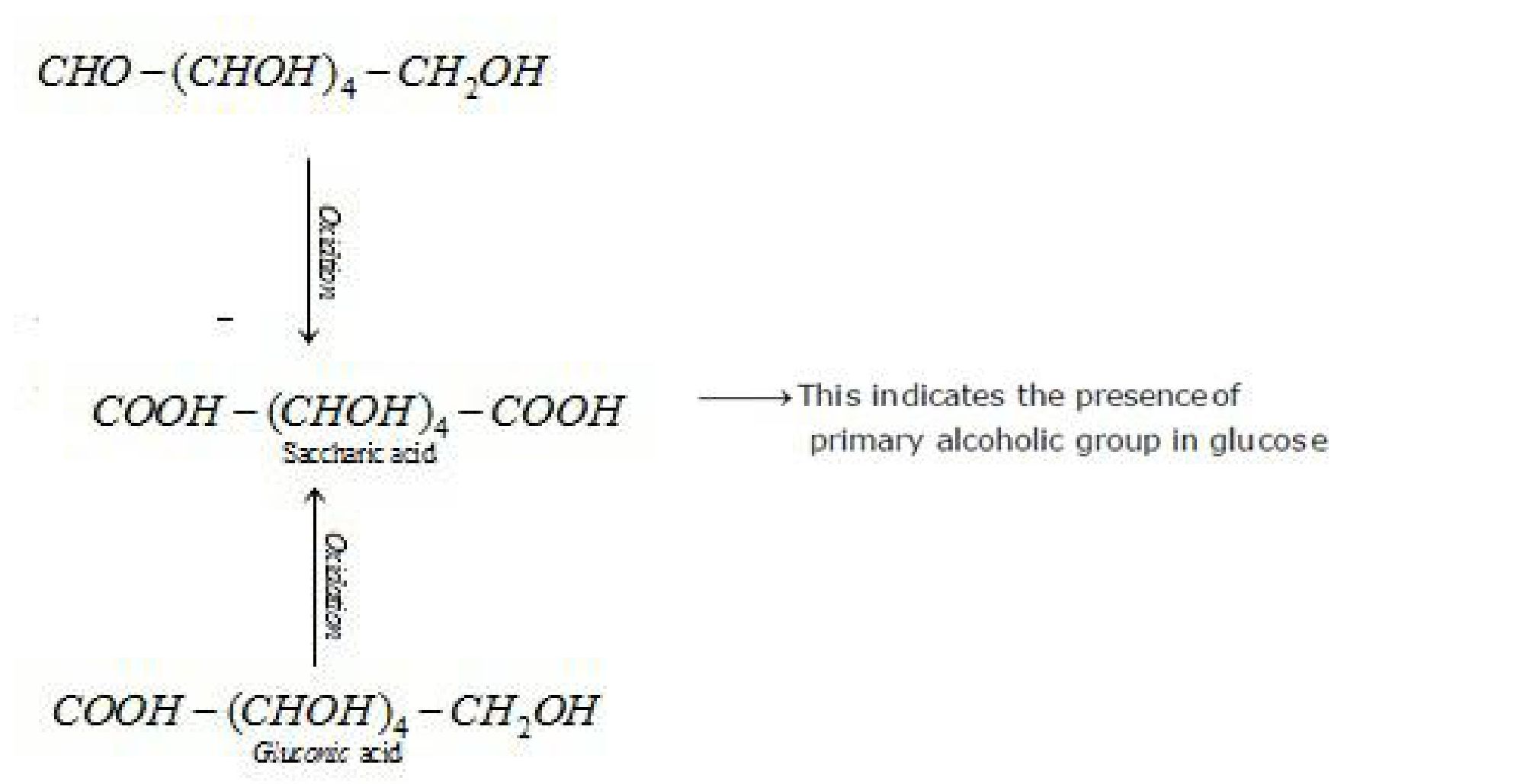
f) D – glucose with nitric acid
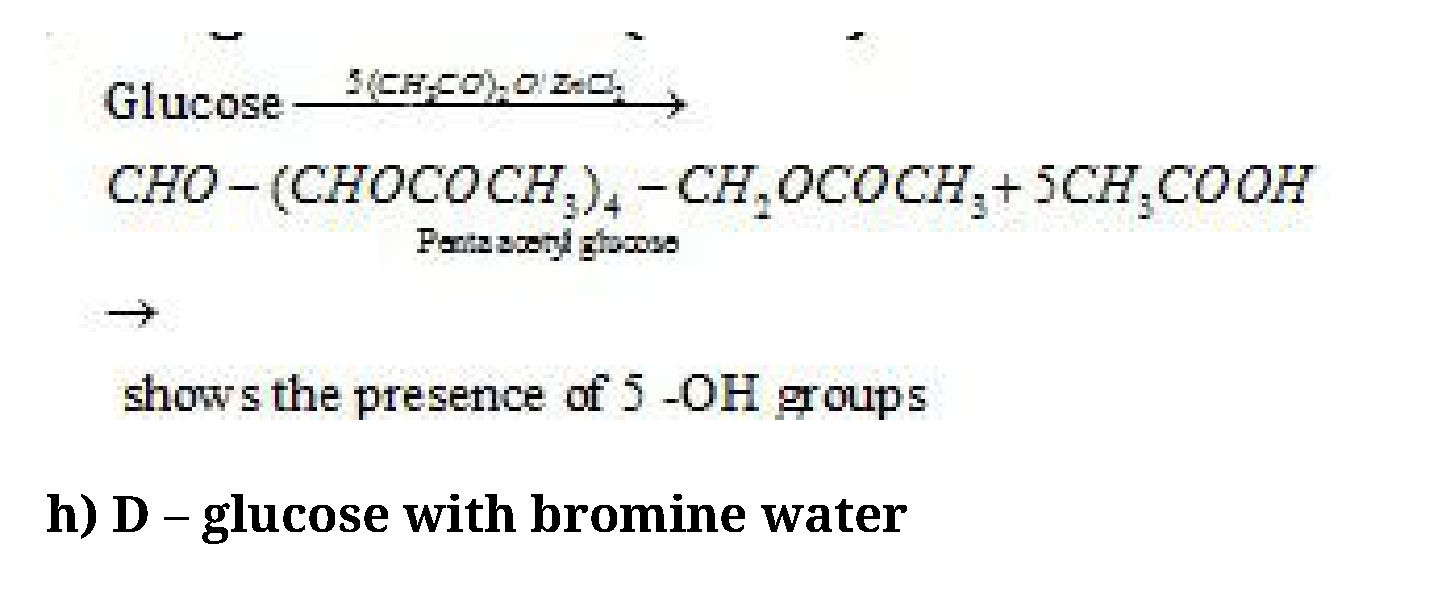
/ 15
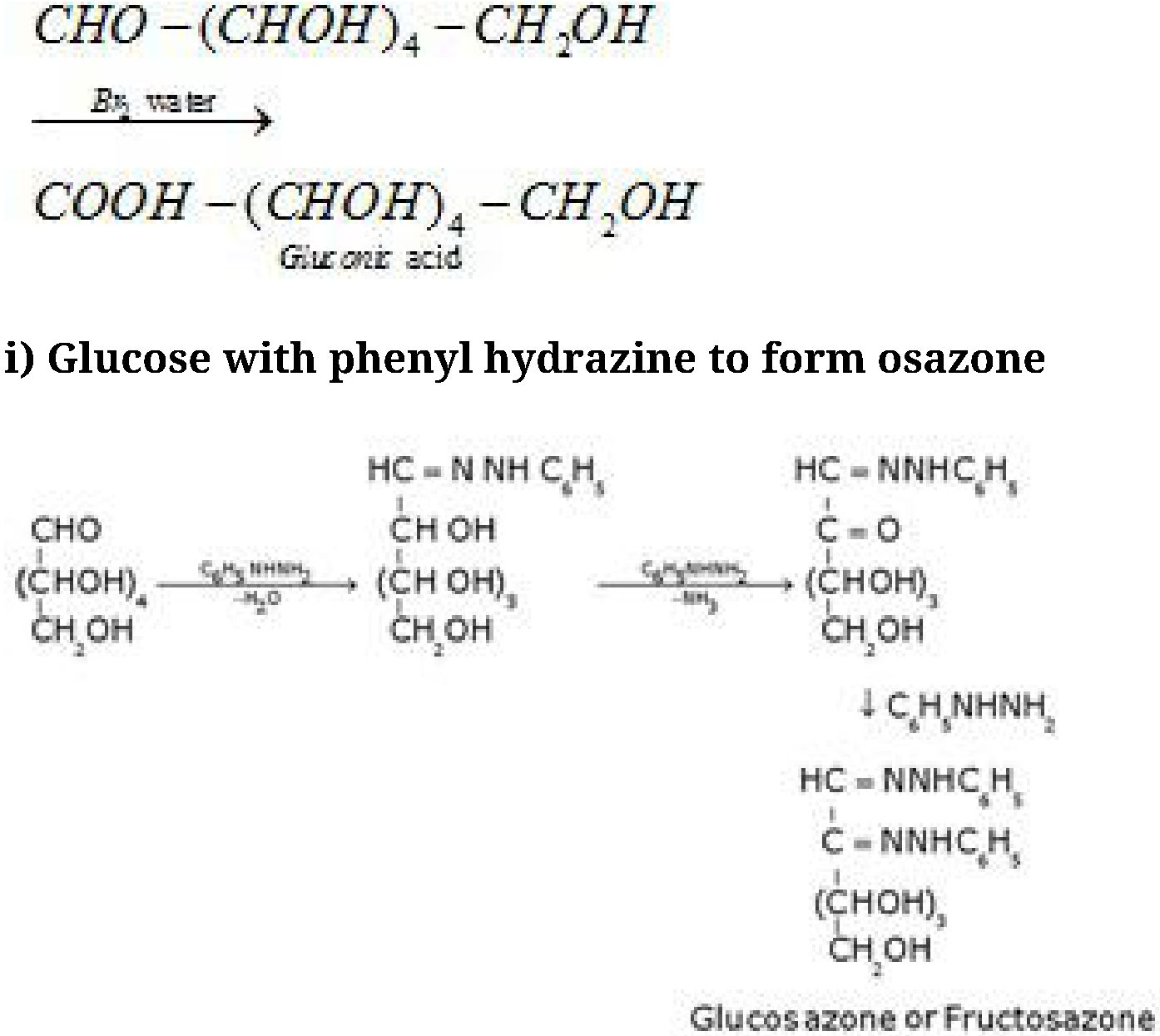
Glucose and fructose gives the same osazone because the reaction takes place at C1 and C2
only.
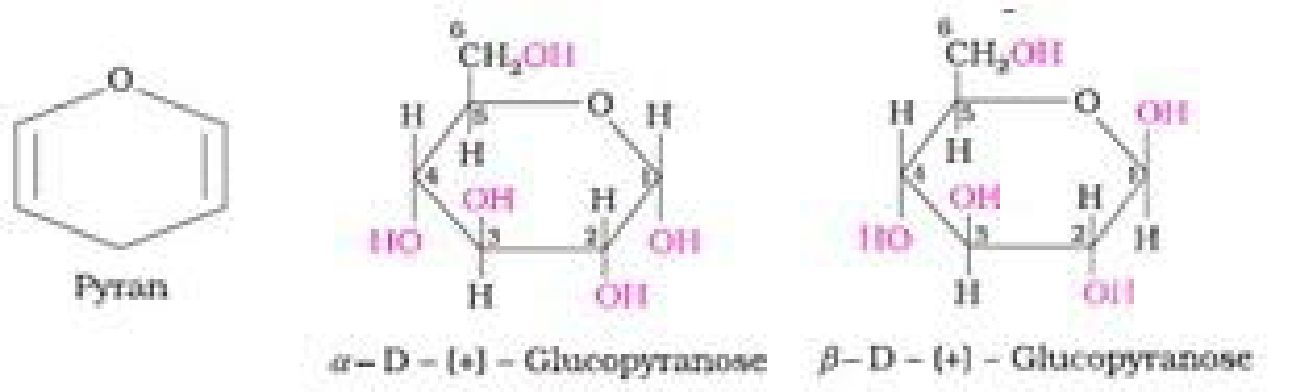
Other Reactions of Glucose (Presence of ring structure)
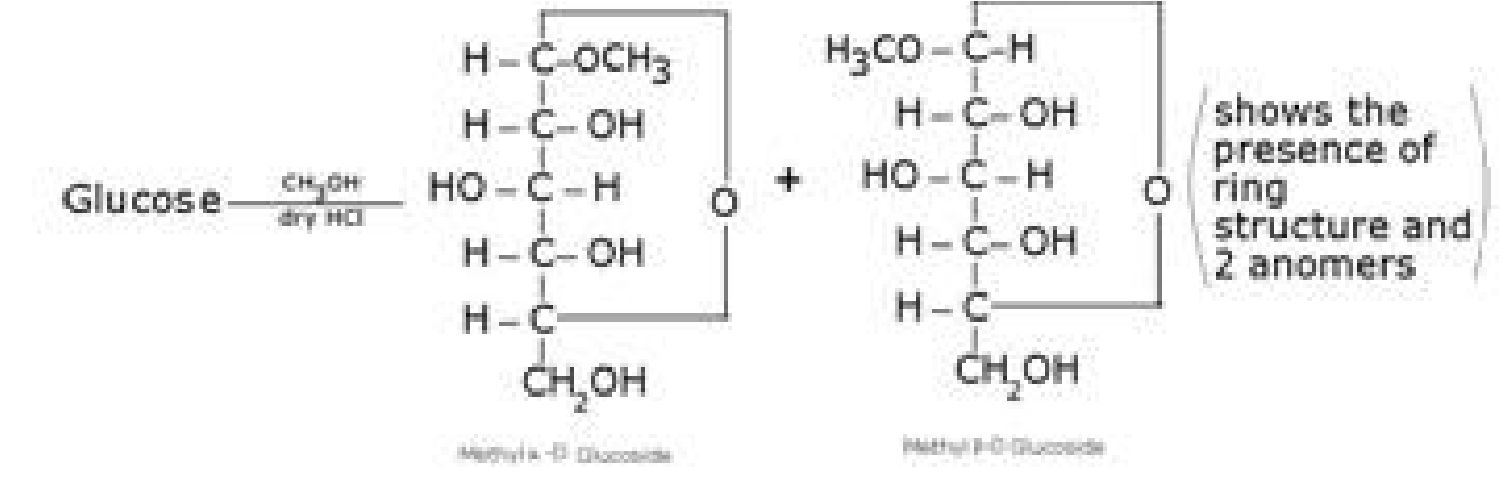
Glucose does not give Schiffs test and does not react with sodium bisulphite and NH3.
Pentaacetyl glucose does not react with hydroxyl amine. This shows the absence of -CHO
group and hence the presence of ring structure.

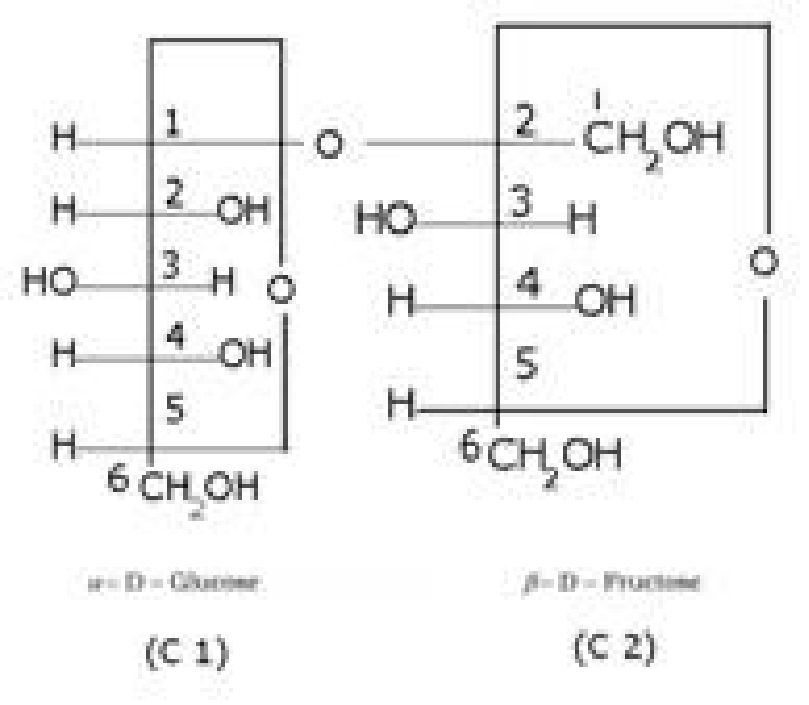
• Haworth representation of glucose:
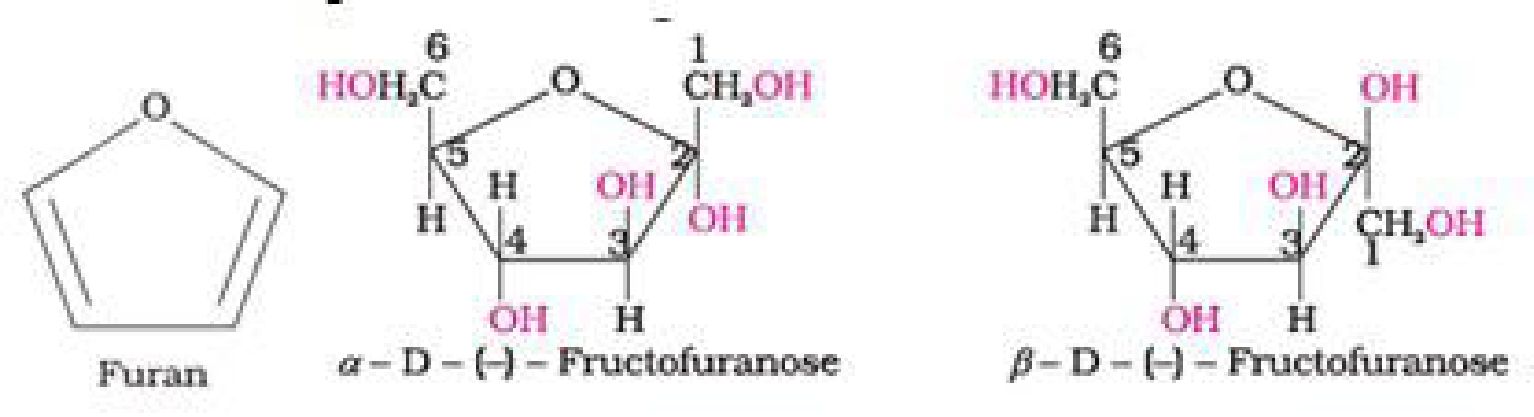
Cyclic structure of fructose:
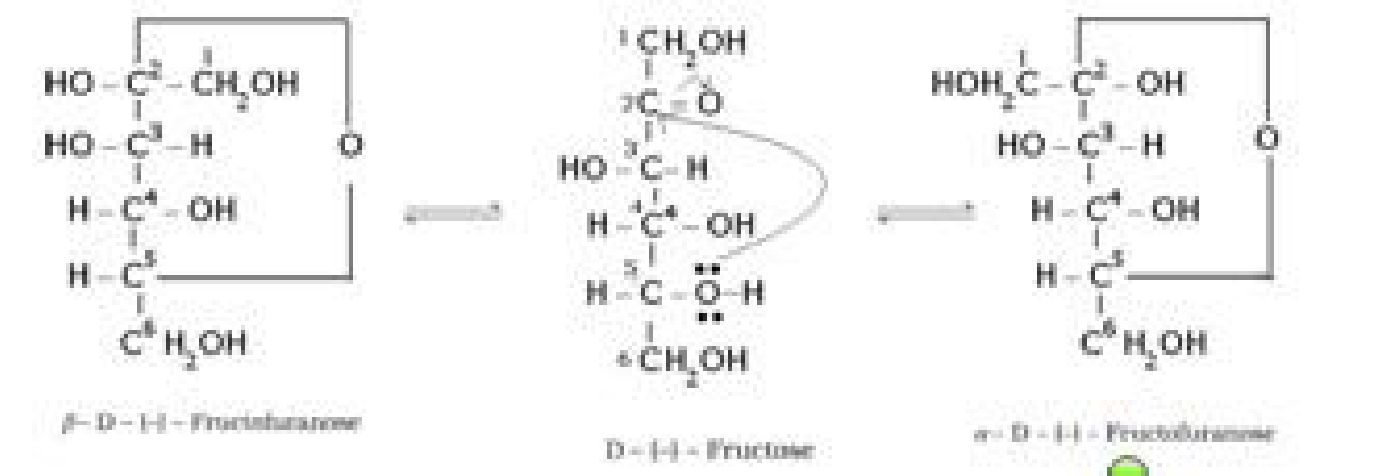
• Haworth representation of fructose
^CiHu°i+C‘Ru0–
D-%izcon D- r>urtare
[*.;.,-+:V^ IafcrJH^
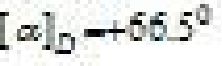
• Haworth Projection of Sucrose:
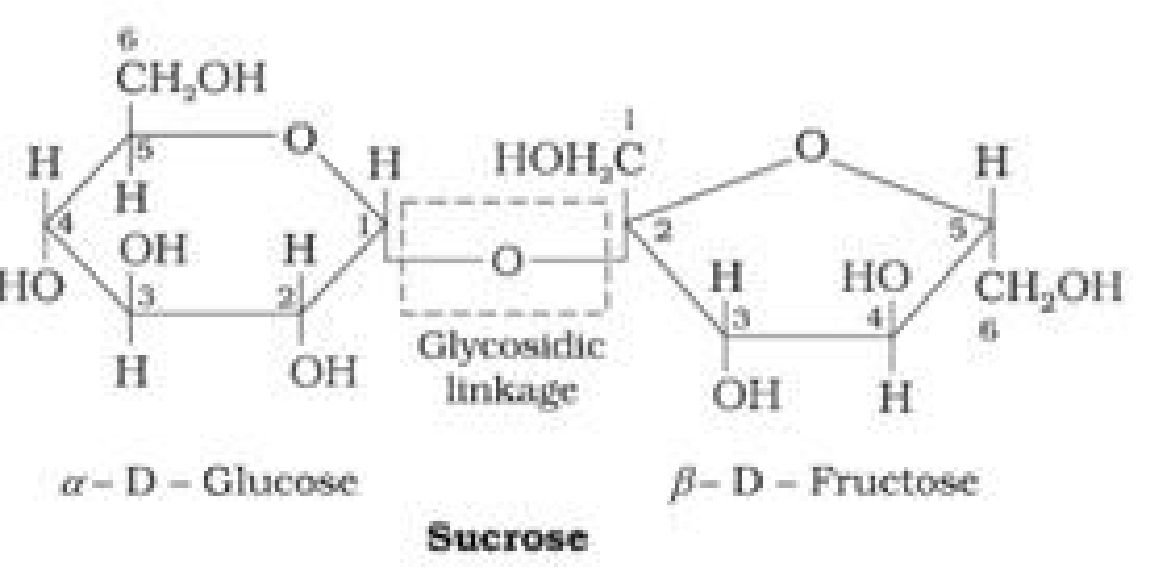

• Haworth projection of maltose:

• Lactose (Milk sugar):It is composed of p-D-galactose and p-D-glucose. The linkage is
between C1 of galactose and C4 of glucose. Hence it is also a reducing sugar.
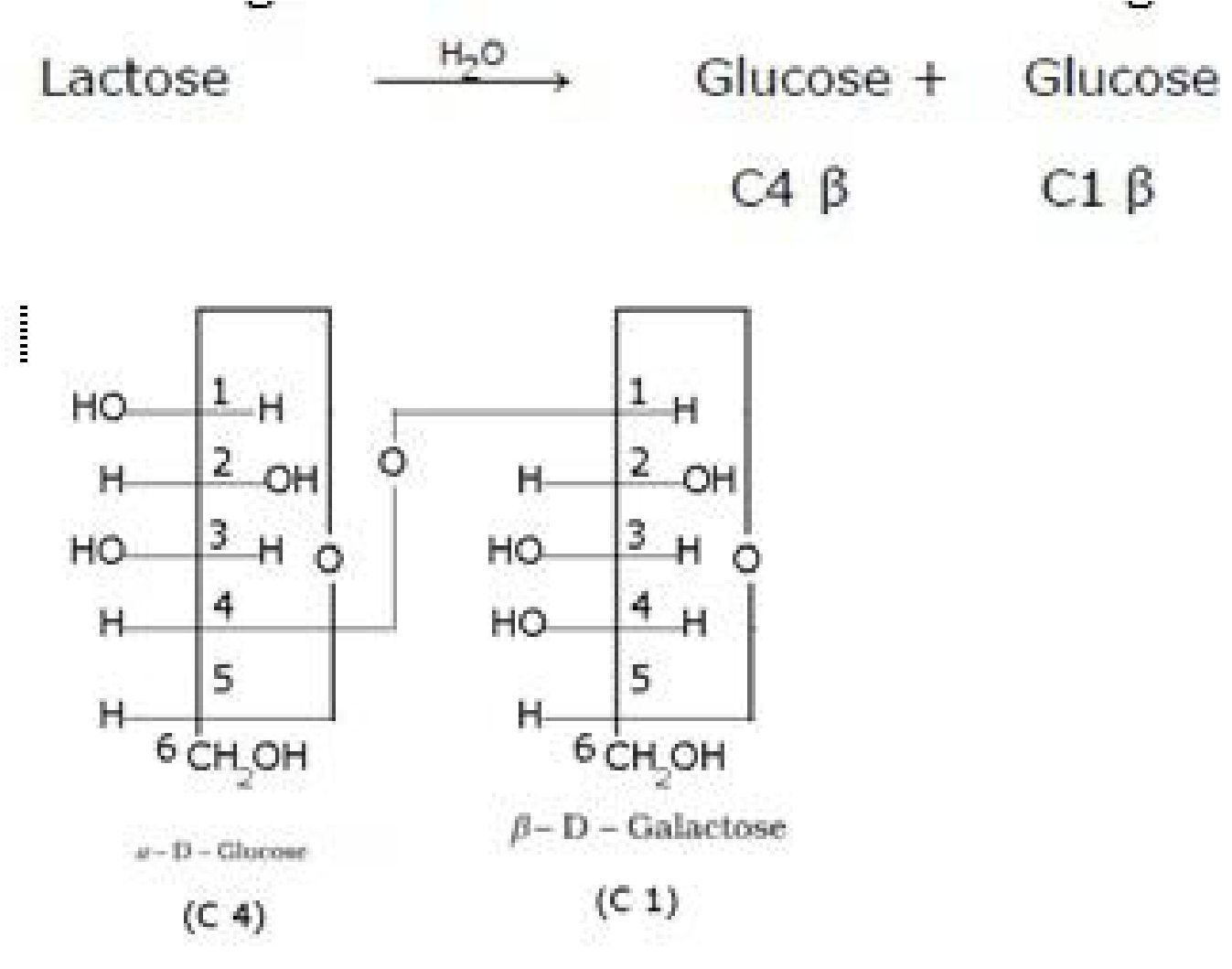
• Haworth projection of lactose:

Amino acids contain amino (-NH2) and carboxyl (-COOH) functional groups.
L
Where R – Any side chain
Most naturally occurring amino acids have L – Config.
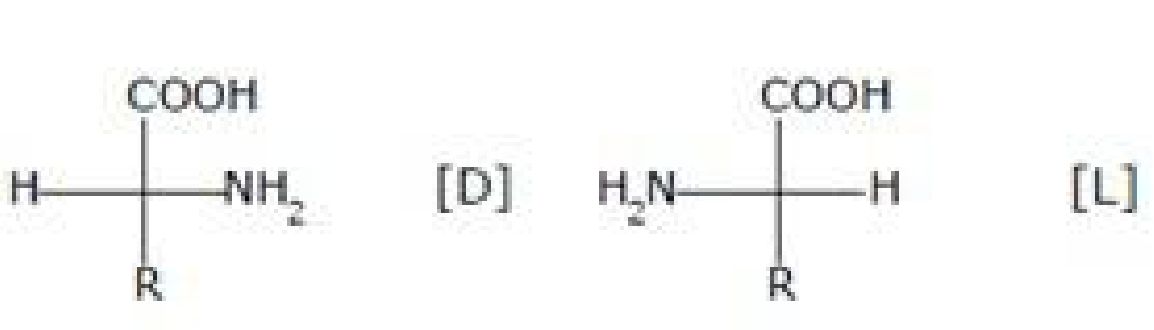
• Types of amino acids:
a). Essential amino acids: The amino acids which cannot be synthesised in the body and
must be obtained through diet, are known as essential amino acids. Examples: Valine,
Leucine
• Zwitter ion form of amino acids:
O O
R-CH-C-O-H^R- CH-C-O-
jffiR W1
2 j
■JSi iiii^ ioa^
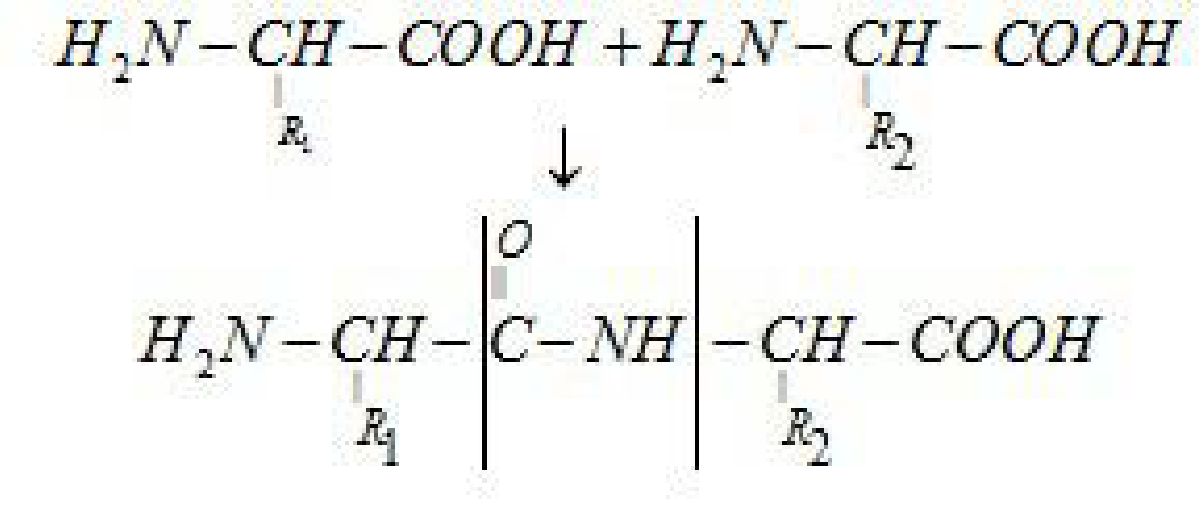
Peptide link age
a- Helix:
and a 13 – membered ring is formed by H – bonding.
/3- pleated sheet:
1. Base + sugar
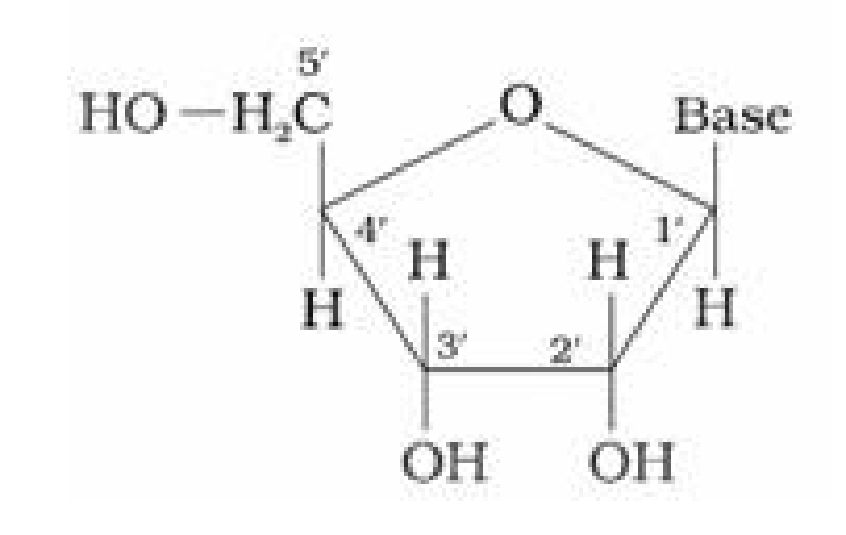
1. Base + sugar + phosphate group

formed between specific pairs of bases.
• Important vitamins, their sources and their deficiency diseases:
|
Name of |
Sources |
Deficiency diseases |
|
Vitamin A |
Fish liver oil, |
xerophthalmia |
|
Vitamin B1 |
Yeast, milk, green |
Beriberi (loss of appetite, retarded growth) |
|
Vitamin B2 |
Milk, egg white, liver, |
Cheilosis (fissuring at corners of mouth and lips), digestive |
|
Vitamin B6 |
Yeast, milk, egg yolk, |
Convulsions |
|
Vitamin B12 |
Meat, fish, egg and curd |
Pernicious anaemia (RBC deficient in haemoglobin) |
|
Vitamin C |
Citrus fruits, amla and |
Scurvy (bleeding gums) |
|
Vitamin D |
Exposure to sunlight, fish |
Rickets (bone deformities in children) and (soft bones and joint pain in adults) |
|
Vitamin E |
Vegetable oils like wheat |
Increased fragility of RBCs and |
|
Vitamin K |
Green leafy vegetables |
Increased blood clotting time |
• Maltose:
Maltose is composed of two a-D-glucose units in which C1 of one glucose (I) is linked to C4
of another glucose unit (II).
The free aldehyde group can be produced at C1 of second glucose in solution and it shows
reducing properties so it is a reducing sugar.
Sucrose (invert sugar): ↑
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 13
Amines
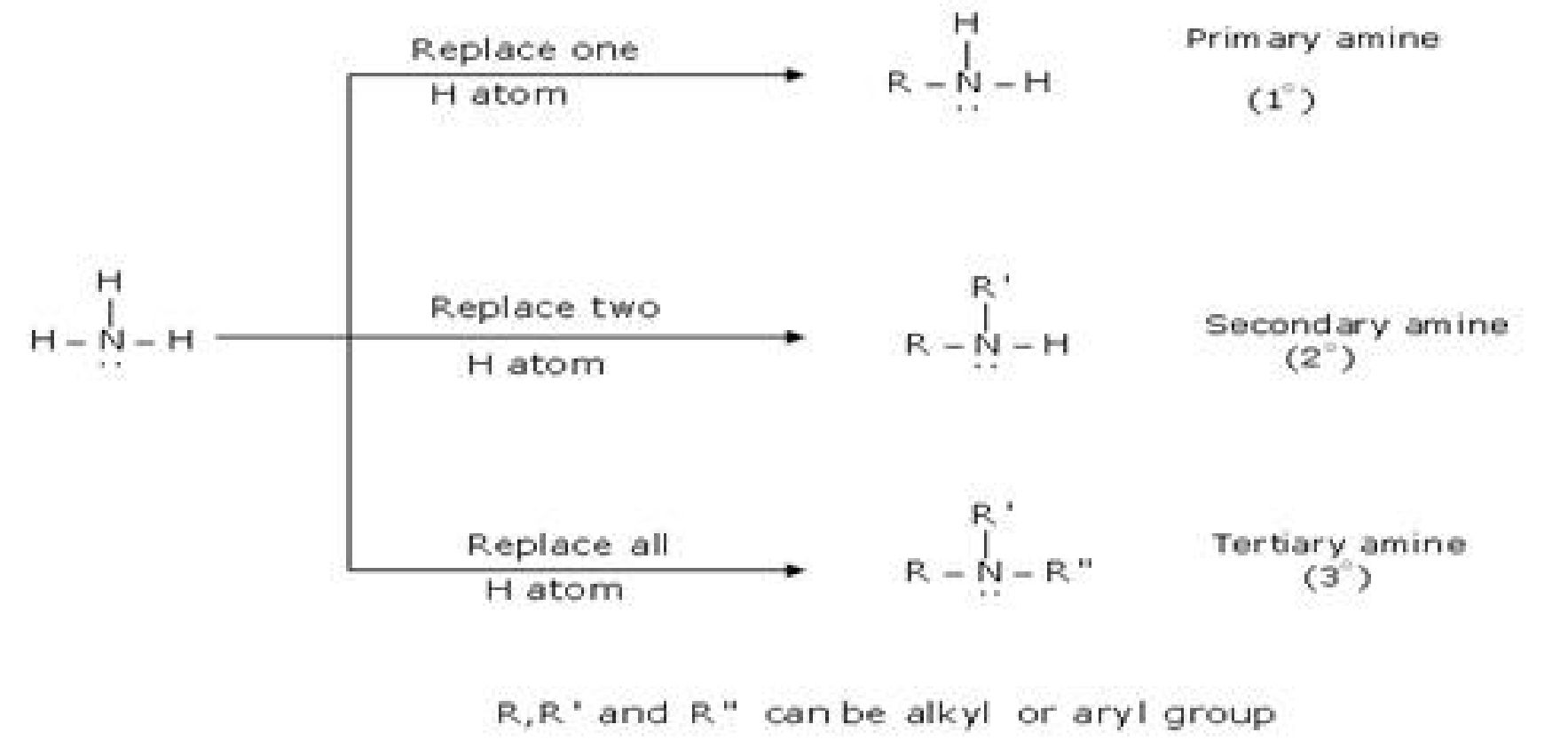
• Amines: Amines are regarded as derivatives of ammonia in which one, two or all
three hydrogen atoms are replaced by alkyl or aryl group.
• Classification of amines:
(i) By reduction of nitro compounds: Nitro compounds can be catalytically reduced by
passing hydrogen gas in presence of Raney Ni, finely divided Pt or Pd as catalyst at room
temperature.
Ni,Pt or pd
a)
Ni, Pt or pd
b)
Nitro compounds can also be reduced with active metals such as Fe, Sn, Zn etc. with conc.
HCl.
Sn/HCl or Fe/HCl
a)
Sn/HCl or Fe/HCl
(ii) By Hoffmann’s method (Ammonolysis of alkyl halides): Reaction of alkyl halides with
an ethanolic solution of ammonia in a sealed tube at 373 K forms a mixture of primary,
secondary and tertiary amine and finally quarternary ammonium salt. Process of cleavage of
C-X bond by ammonia is called ammonolysis.
+■ —
RNH2 — > R2NH f–l: > R3N K>: > S4 A7 X
(£) (2e) (je) Q*at9T#nrj/.
3Tjy.PT.LUTL E-L |[ I
NaOH
a)
(l°a min e)
NaOH
b)
(2°amine)
NaOH
c)
(3° a min e)
Method is not suitable for preparation of aryl amines because aryl amines are relatively less
reactive than alkyl halides towards nucleophilic substitution reactions.
H2/Ni
Or
Na(Hg)/C2H5OH
Or
LiAlHt

Aromatic primary amines cannot be prepared by this method because aryl halides do not
undergo nucleophilic substitution with potassium phthalimide.
parent amide.
0
Il
i
R – NH2 + Na2 CO2 + 2 NaBr + 2 H2O
Physical properties of amines:
• Chemical properties of amines:
[.R-NHz][OH]
[R-NH2][H20\
[R-NHs][OH]
Or K[H20] =
[R-NH2]
_ [R-NH3][OH]
b [R-NH2]
pKb = -Iogif6
Greater Kb value or smaller pKb indicates base is strong.
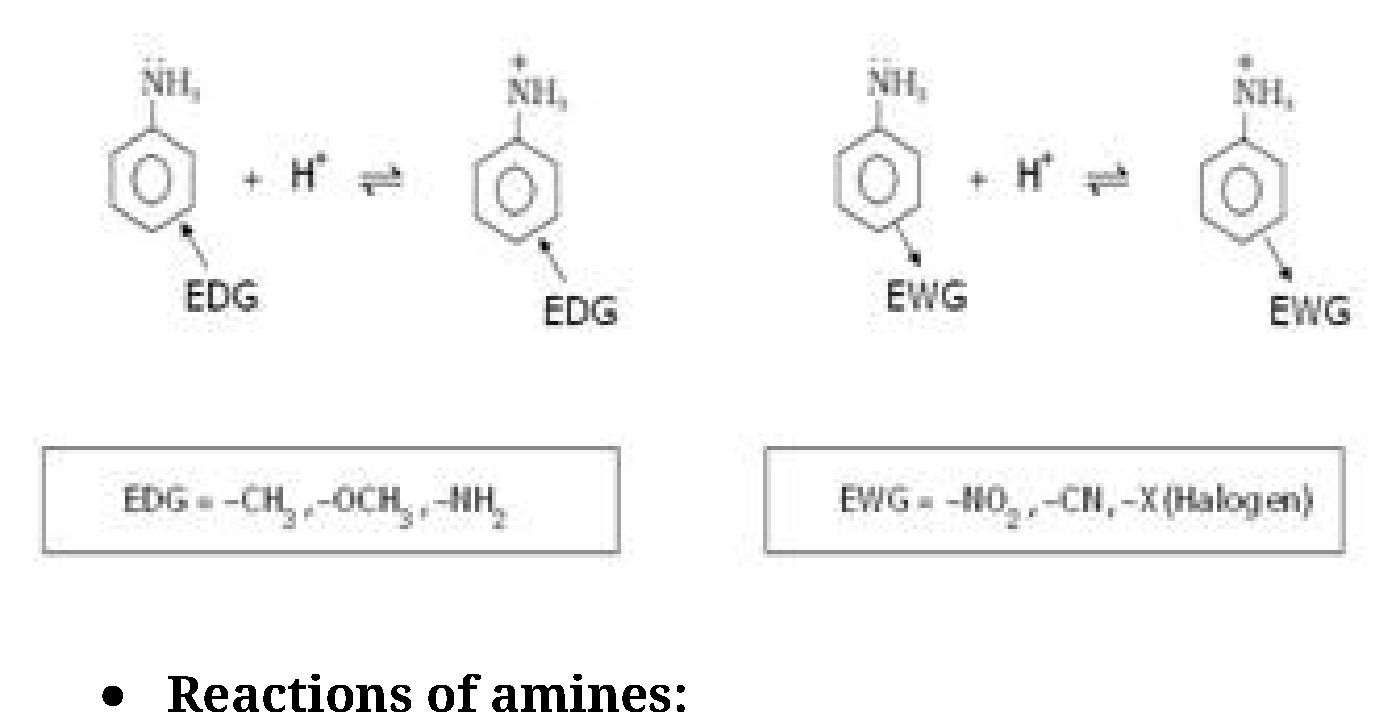
(i) The order of basicity of amines in the gaseous phase follows the expected order on the
basis of +I effect: tertiary amine > secondary amine > primary amine > NH3
(ii) In aqueous solution it is observed that tertiary amines are less basic than either primary
or secondary amines. This can be explained on basis of following factors:
On the basis of solvation effect order of basicity of aliphatic amines should be primary
amine>secondary amine>tertiary amine.
this case order of basicity in aqueous medium is
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
When alkyl group is ethyl group order of basicity in aqueous medium is
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
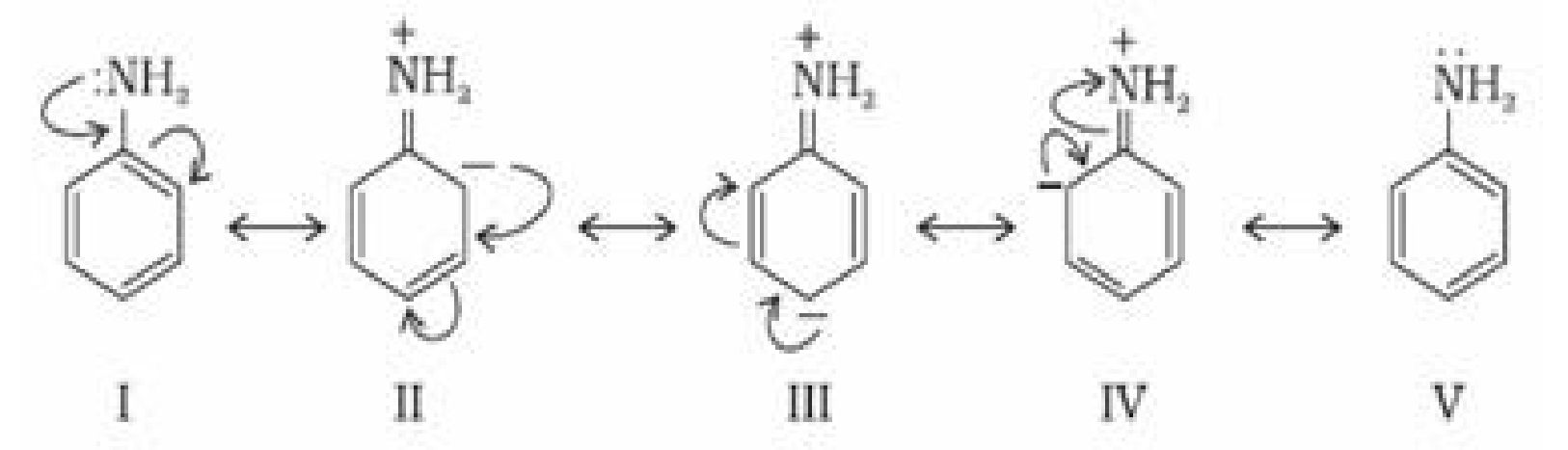
In the above resonating structures there is a positive charge on nitrogen atom making the
lone pair less available for protonation. Hence aniline is less basic than ethyl amine which
has no resonating structures. Less basicity of aniline can also be explained by comparing the
relative stability of aniline and anilinium ion obtained by accepting a proton. Greater the
number of resonating structures, greater is the stability of that species.
Aniline is resonance hybrid of five resonating structures whereas anilinium ion has only two
resonating structures.
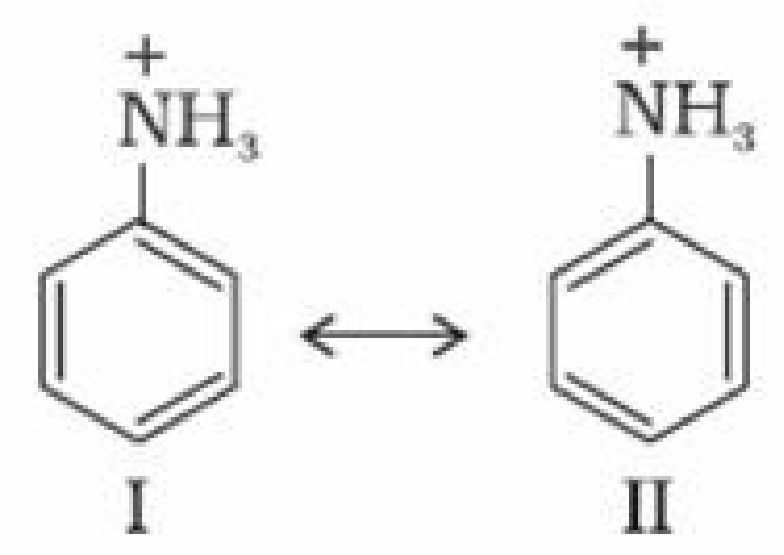
Thus aniline has less tendency to accept a proton to form anilinium ion.
a) Acylation Reaction: Aliphatic and aromatic primary and secondary amines (which
contain replaceable hydrogen atoms) react with acid chlorides, anhydrides and esters to
form substituted amide. Process of introducing an acyl group (R-CO-) into the molecule is
called acylation. The reaction is carried out in the presence of a stronger base than the
amine, like pyridine, which removes HCl formed and shifts the equilibrium to the product
side.
Base
R – NH2 + RCOCl > RNHCOR + HCl
Add chloride Substituted amide
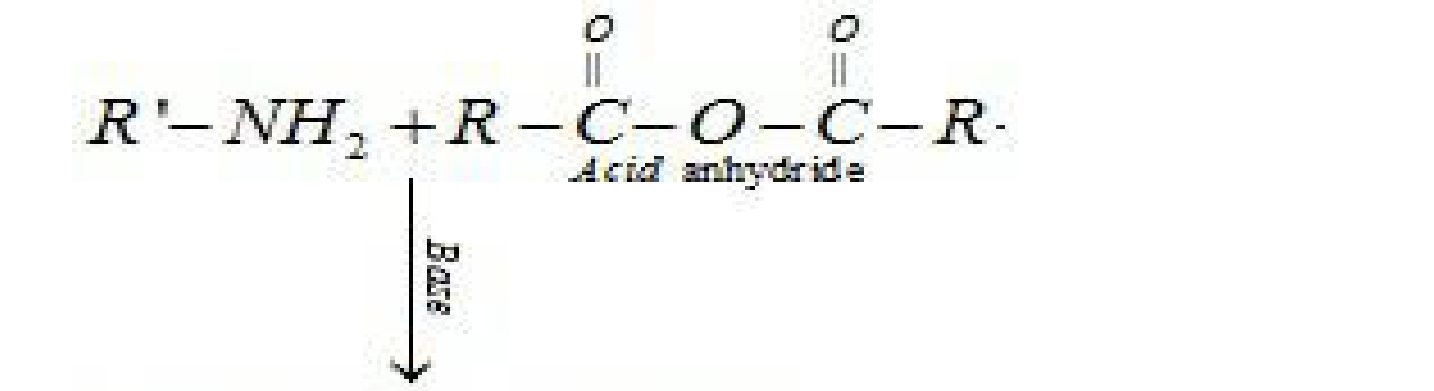
^Li bzmwed em^
Since tertiary amine do not contain replaceable hydrogen atom they do not undergo
acylation reaction.
b) Carbylamine reaction: Only aliphatic and aromatic primary amines on heating with
chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines.
R – NH0 + CHCl + 3 KOH
2. .-
|
1 F
R – NC + 3KCl + ^H2O
Secondary and tertiary amines do not give the above test.
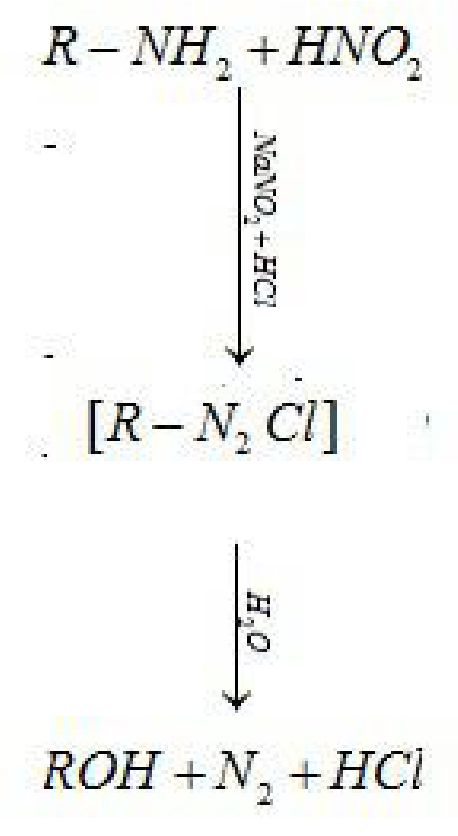
(i) Primary aliphatic amine on reaction with nitrous acid (HNO2) forms aliphatic
diazoniumsalt which decomposes to form alcohol and evolve nitrogen.
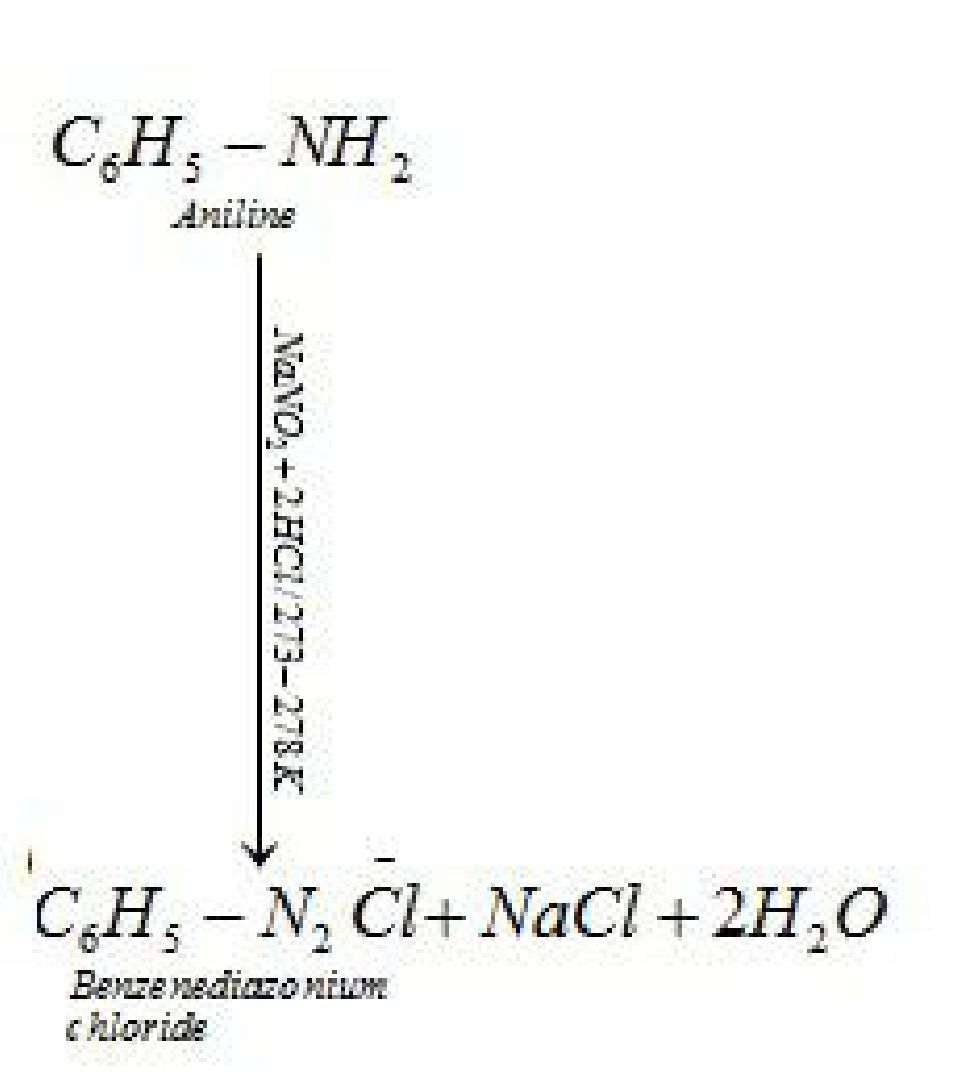
(ii) Primary aromatic amines react with nitrous acid (HNO2) in cold (273-278 K) to form
diazonium salt.
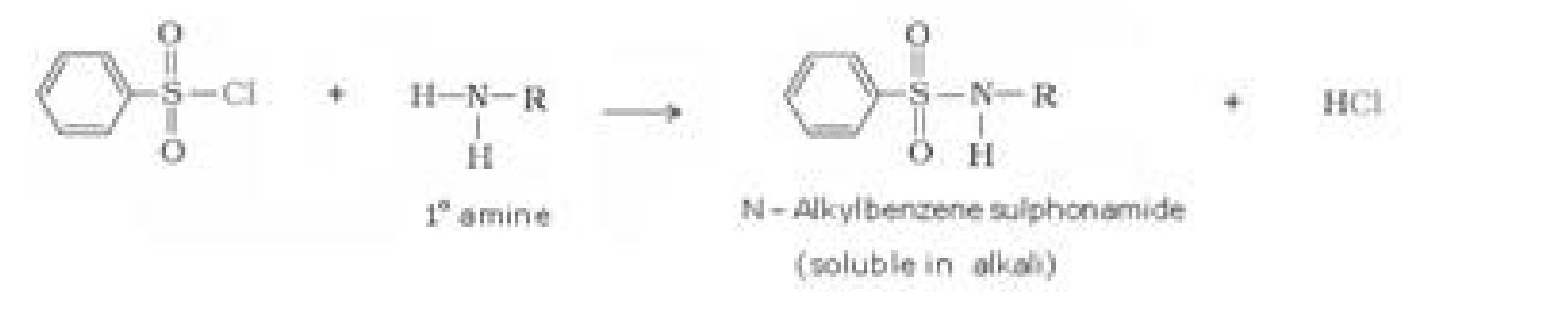
The hydrogen attached to nitrogen in sulphonamide formed by primary amine is strongly
acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is
soluble in alkali.

Since sulphonamide formed by secondary amine does not contain any hydrogen atom
attached to nitrogen atom, so it is not acidic. Hence it is insoluble in alkali.
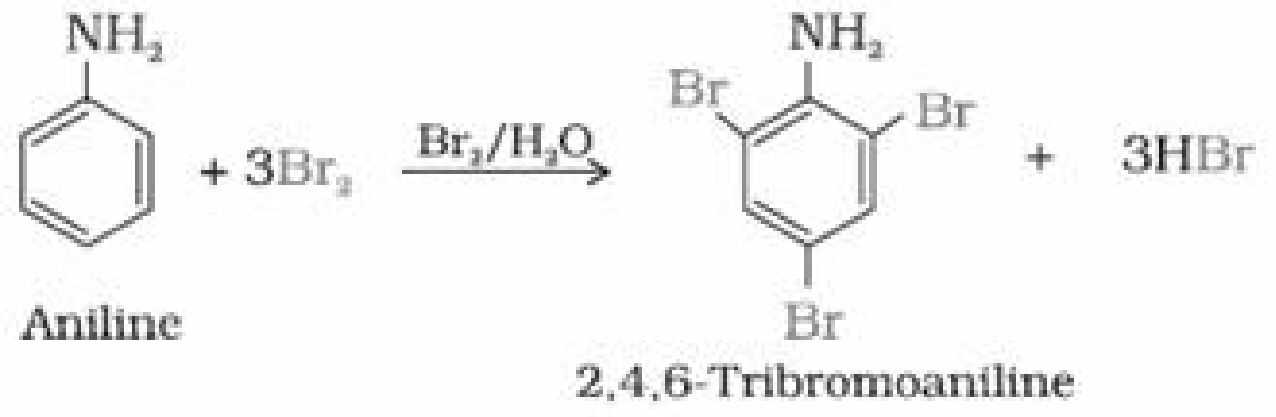
• Ring substitution in aromatic amine: Aniline is more reactive than benzeneand
undergoes electrophilic substitution reaction preferably at ortho and para position.
(i) Bromination: Aniline reacts with bromine water at room temperature to give a white
precipitate of 2, 4, 6-tribromoaniline
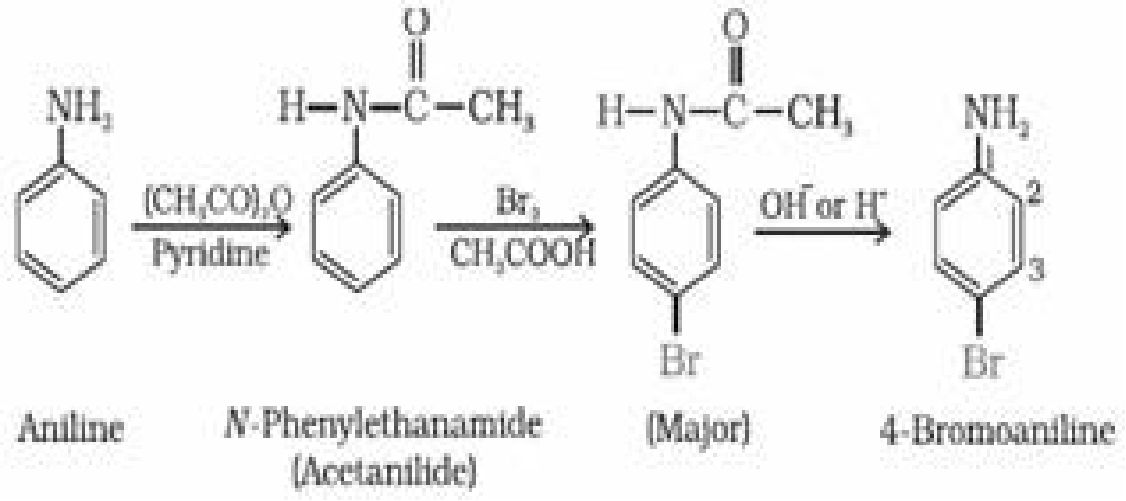
In order to stop reaction at monosubstitution activating effect of -NH2 group is reduced by
acetylation. This prevents di and tri substituted products. Acetyl group is removed by
hydrolysis.
(a) Under strongly acidic medium aniline gets protonated to form anilinium ion, which is
deactivating group and is meta directing. Hence minitroaniline is also formed in 47% along
with ortho and para products.
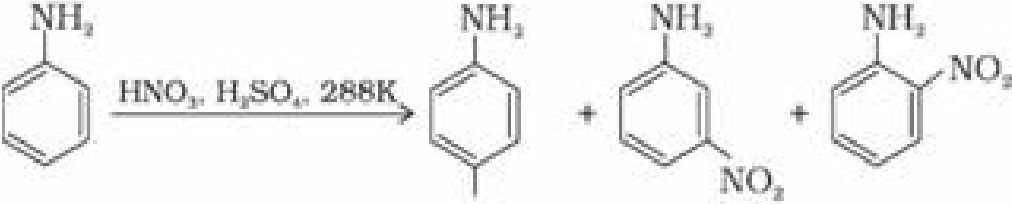
NOj
(51¾) (47%) (2%)
Aromatic amines cannot be nitrated directly because HNO3 being a strong oxidising agent
oxidises it forming black mass.
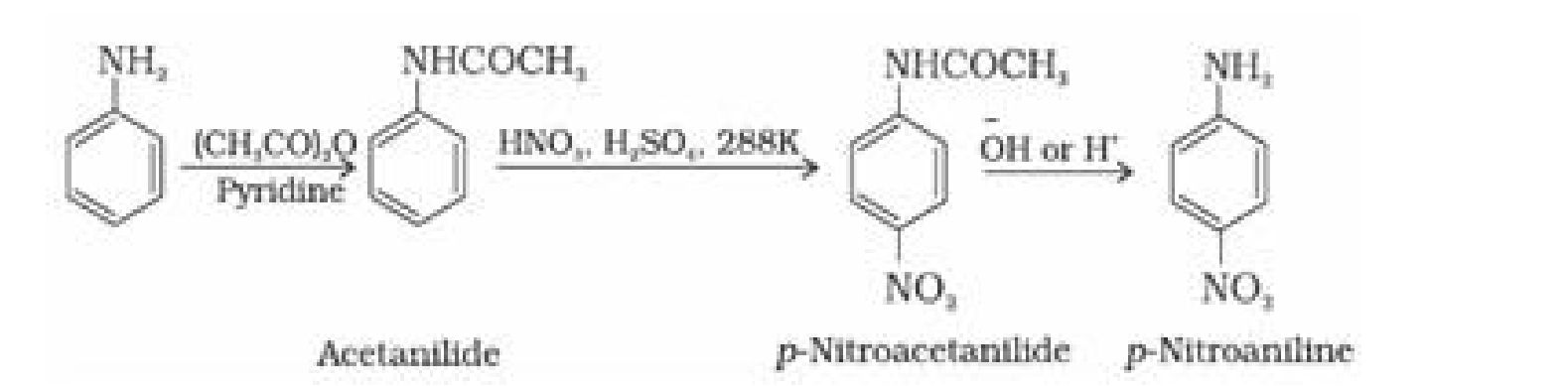
(b) Nitration by protecting the -NH2 group by acetylation reaction with acetic anhydride:
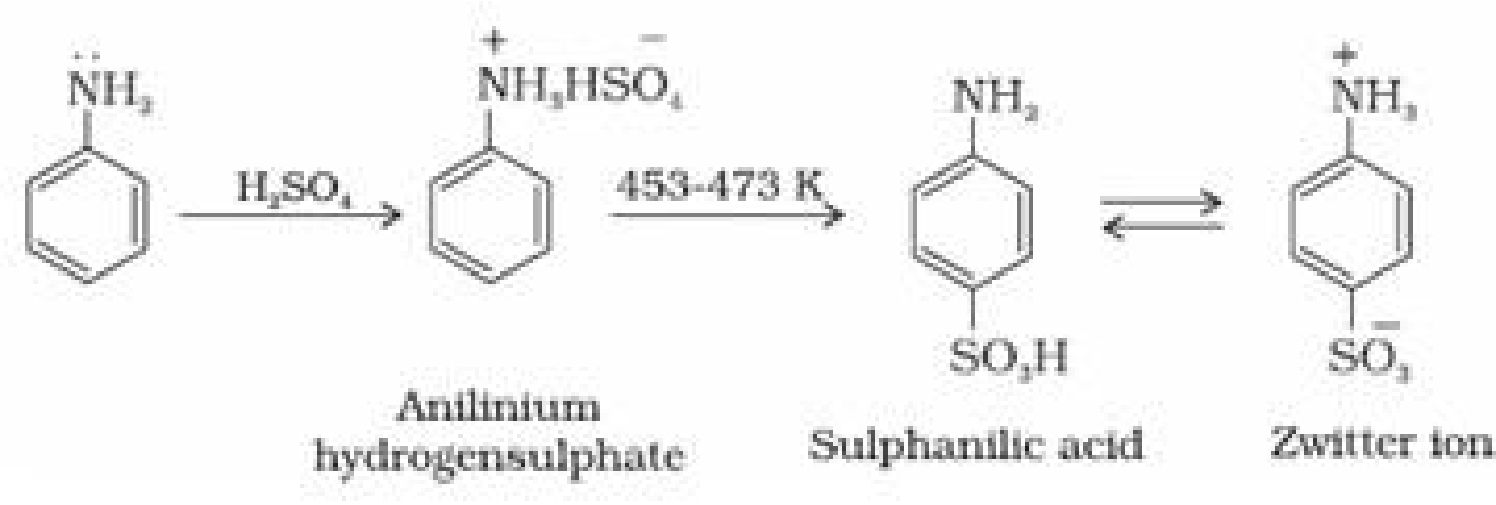
iii) Sulphonation: Aniline reacts with conc. H2SO4 to form aniliniumhydrogensulphate which
on heating with sulphuric acid at 453-473K produces p-aminobenzenesulphonic acid,
commonly known as sulphanilic acid, as the major product.
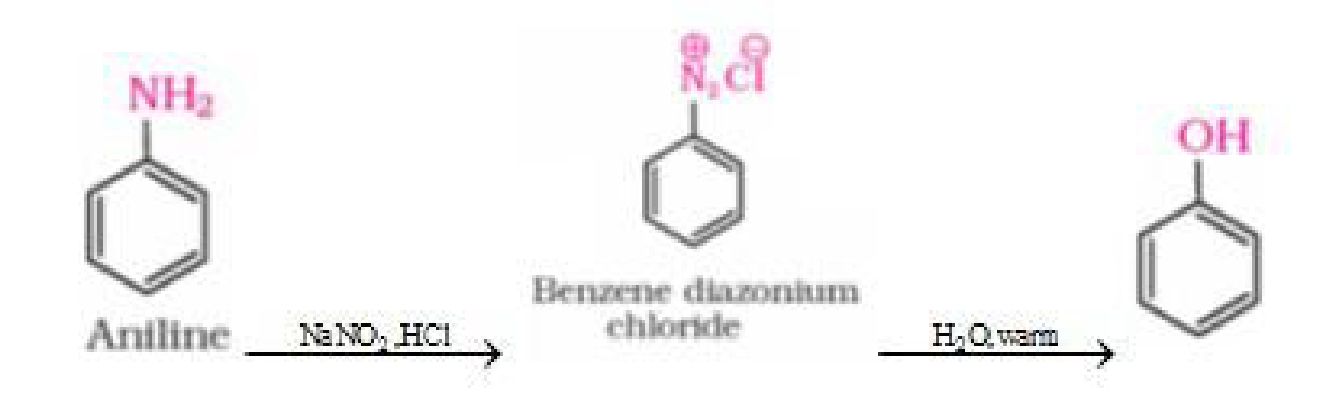
• Reactions ofbenzene diazonium chloride:
a) Reactions involving displacement of nitrogen:
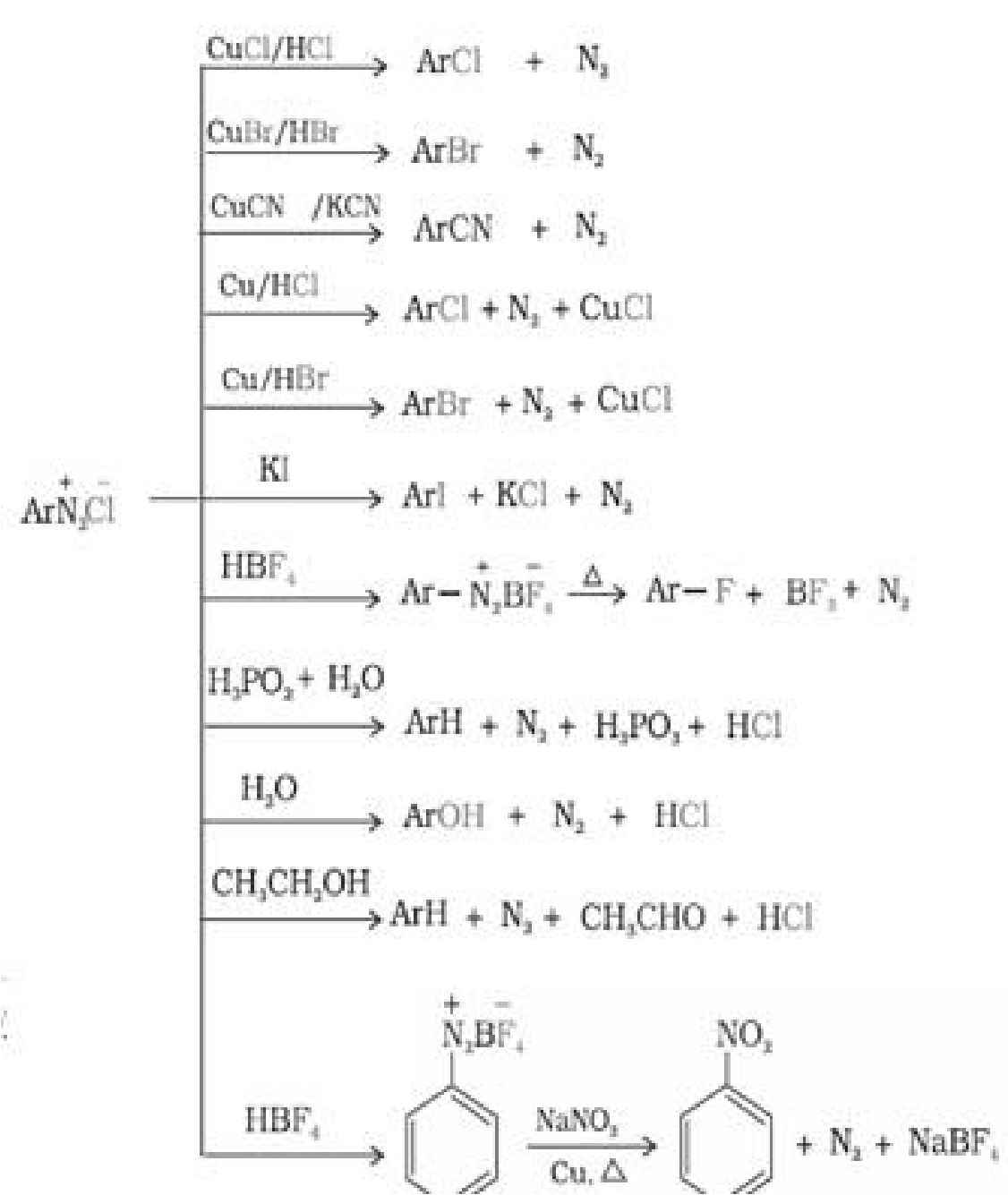
Material Downloaded From SUPERCOP
/ 11
b) Reactions involving retention of diazo group, coupling reactions: Diazonium ion
acts as an electrophile because there is a positive charge on terminal nitrogen.
Therefore benzene diazonium chloride couples with electron rich compounds like
phenol and aniline to give azo compounds. Azo compounds contain -N=N- bond and
reaction is coupling reaction.
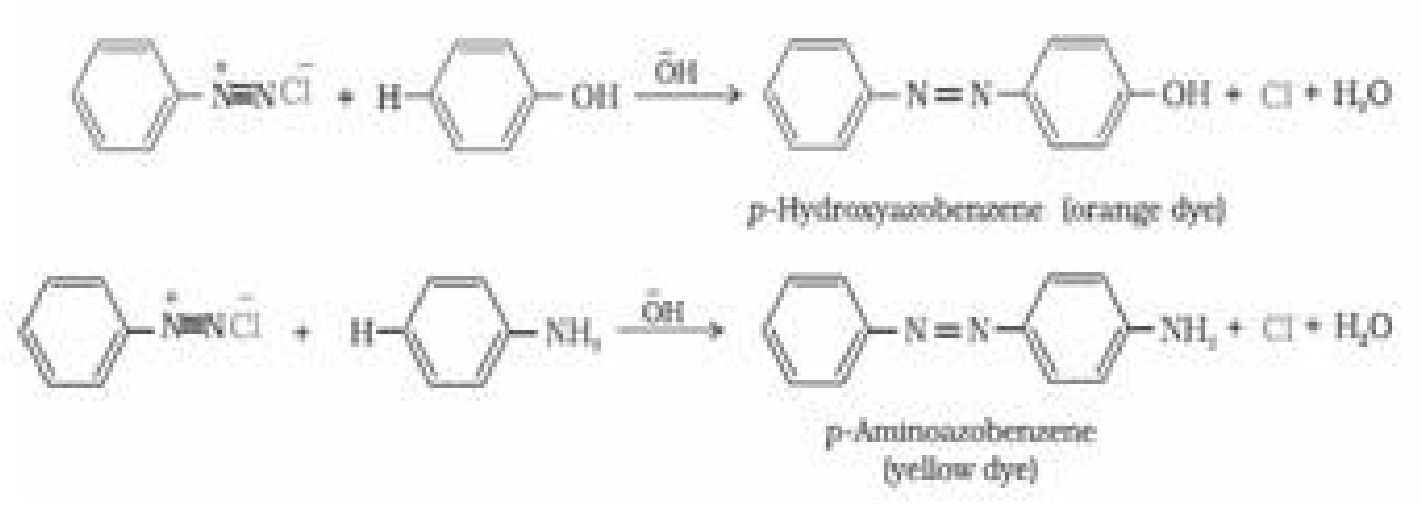
CSBE Class 12 Chemistry
Revision Notes
Chapter 12
Aldehydes, Ketones and Carboxylic acid
Aldehydes: Aldehydes are the organic compounds in which carbonyl group is attached to
one hydrogen atom and one alkyl or aryl group.

Where R can be an alkyl or aryl group

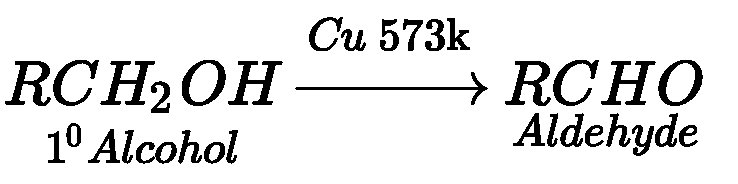
acetaldehyde.

d) By Rosenmund reduction: Hydrogenation of acyl chloride over palladium on barium
sulphate gives aldehyde.
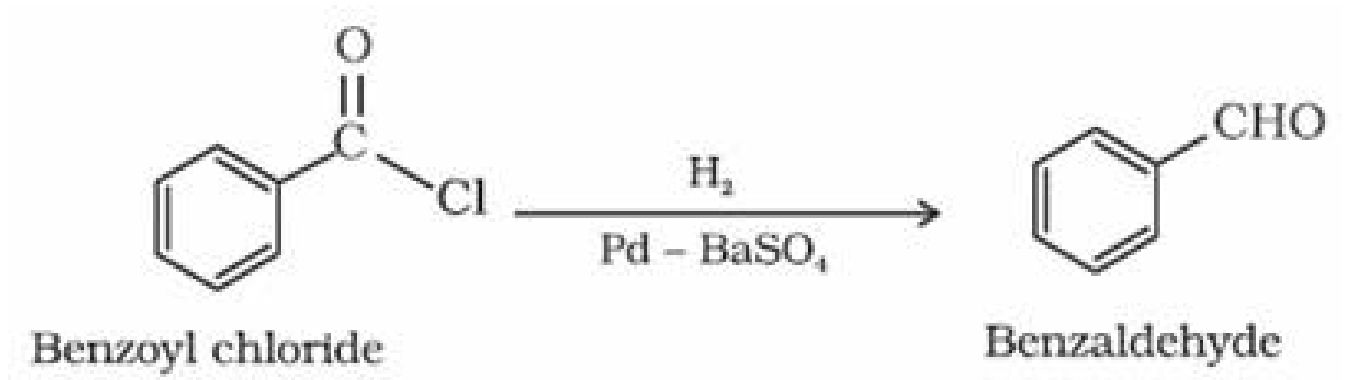
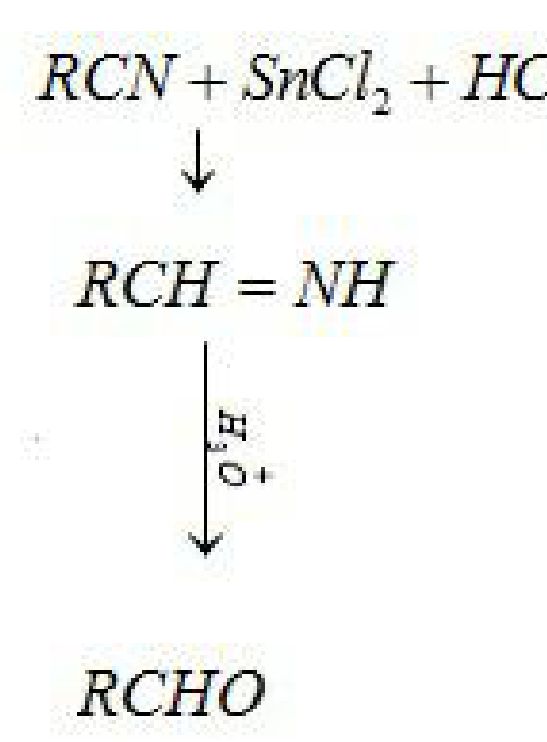
i) Stephen Reaction: Reduction of nitriles in presence of stannous chloride in presence of HCl
gives imine which on hydrolysis gives corresponding aldehyde.
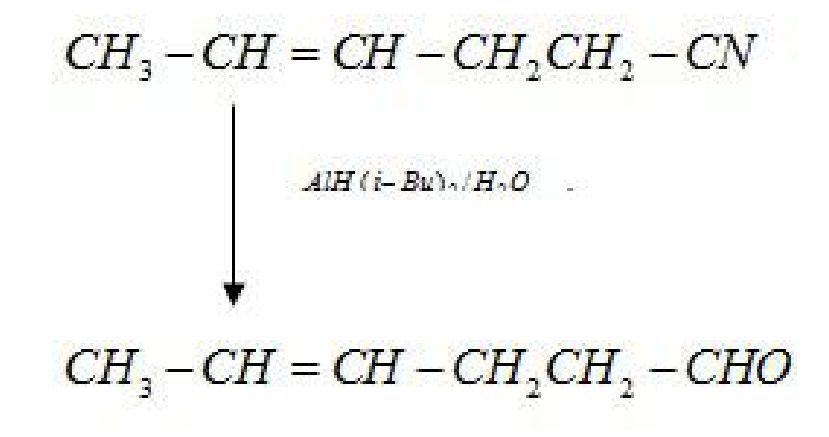
ii) Nitriles are selectively reduced by DIBAL-H (Diisobutylaluminium hydride) to aldehydes.
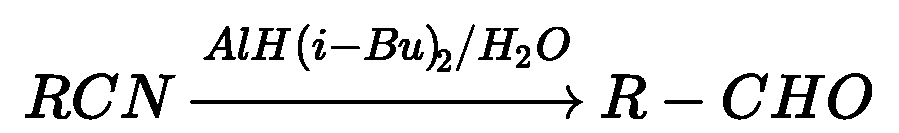
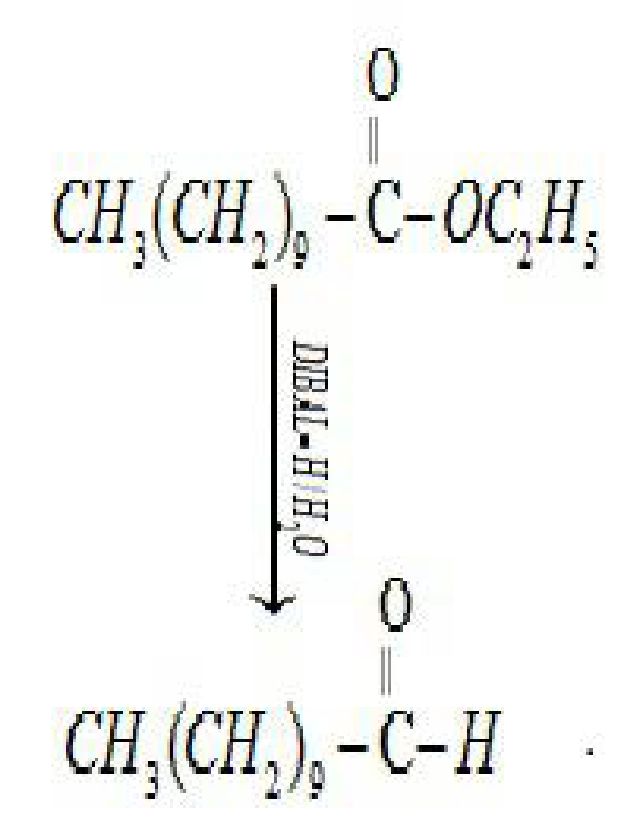
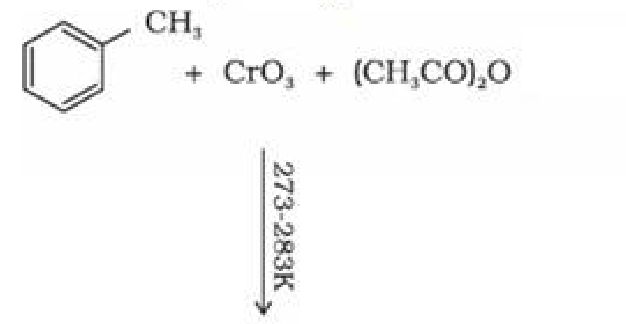
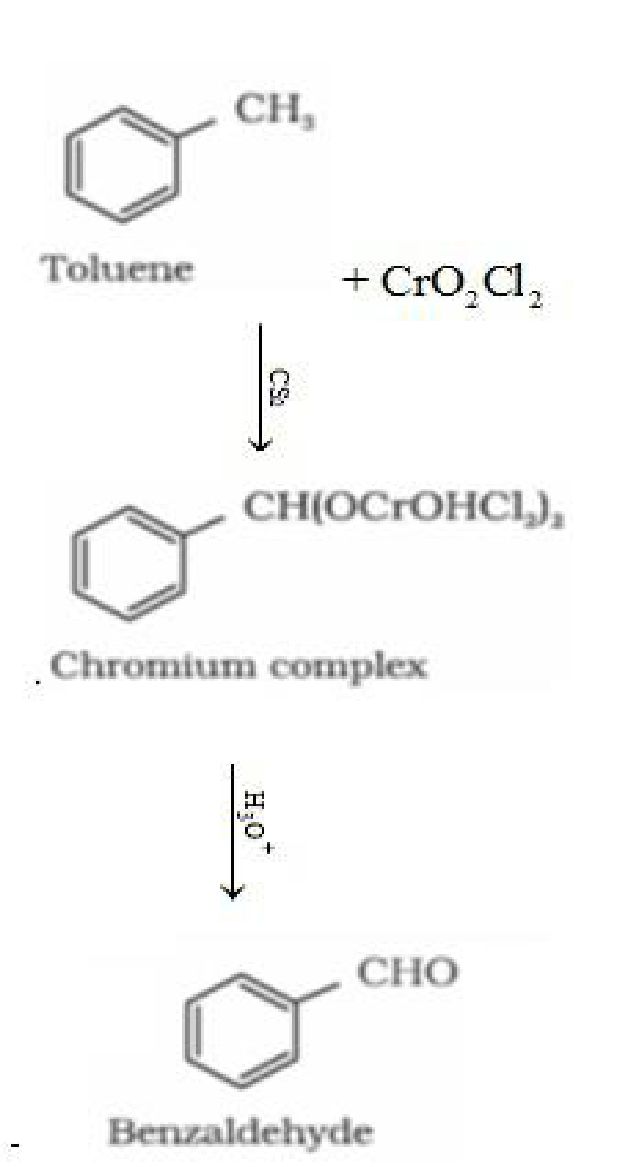
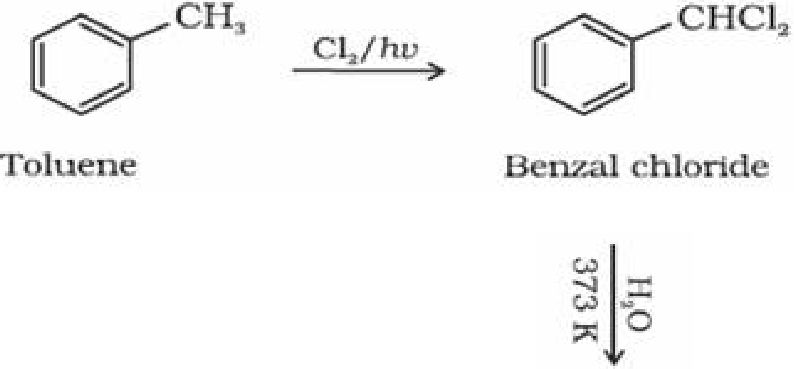
Using chromium oxide(Cr03): Toluene or substituted toluene is converted to benzaldehyde
in presence of chromic oxide in acetic anhydride.


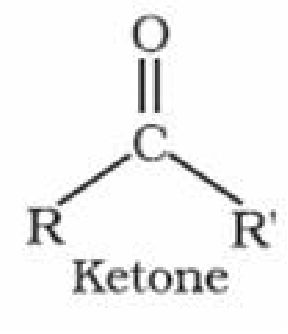
Ketones: Ketones are the organic compounds in which carbonyl group is attached to
two alkyl group or aryl group or both alkyl and aryl group.



l I
C=C —
Propene
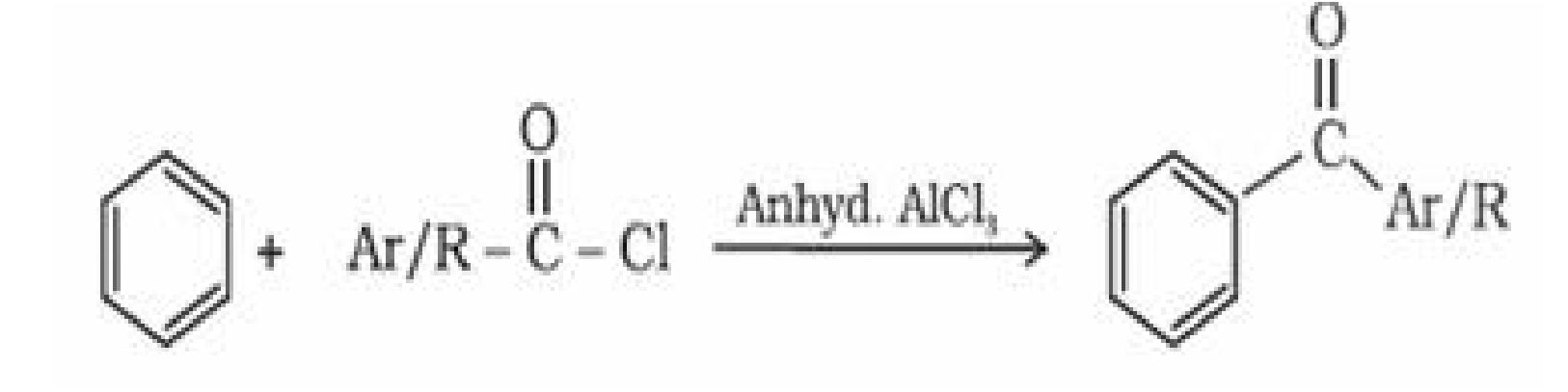
+ O1– ^ —C C —
O — O
| Zn + I ^O
— C = O + O = C —
Ald&bydes orKetones
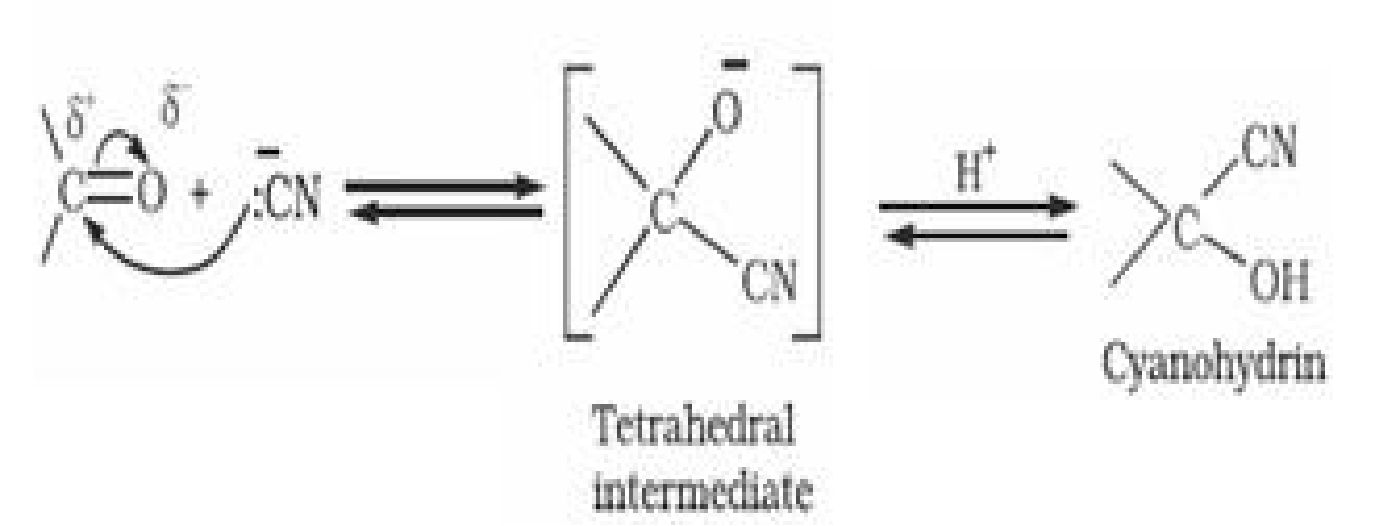
(a) Addition of hydrogen cyanide (HCN) to form cyanohydrins
(b) Addition of sodium hydrogensulphite(^a#,L>O3)to form bisulphate addition compound

(c) Addition of Grignard reagent (RMgX) to form alcohol
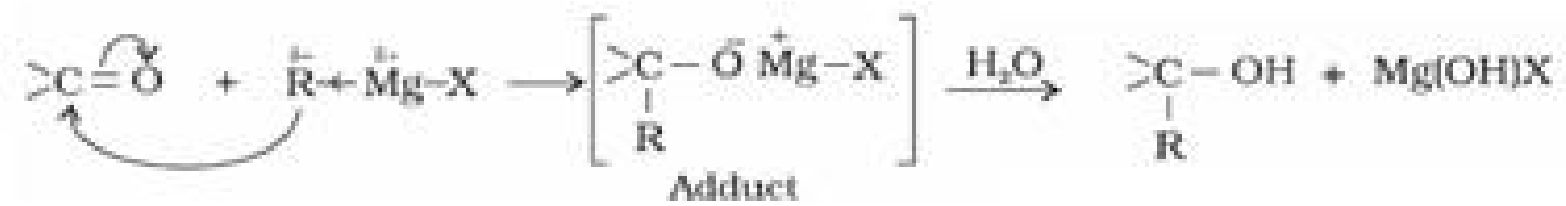
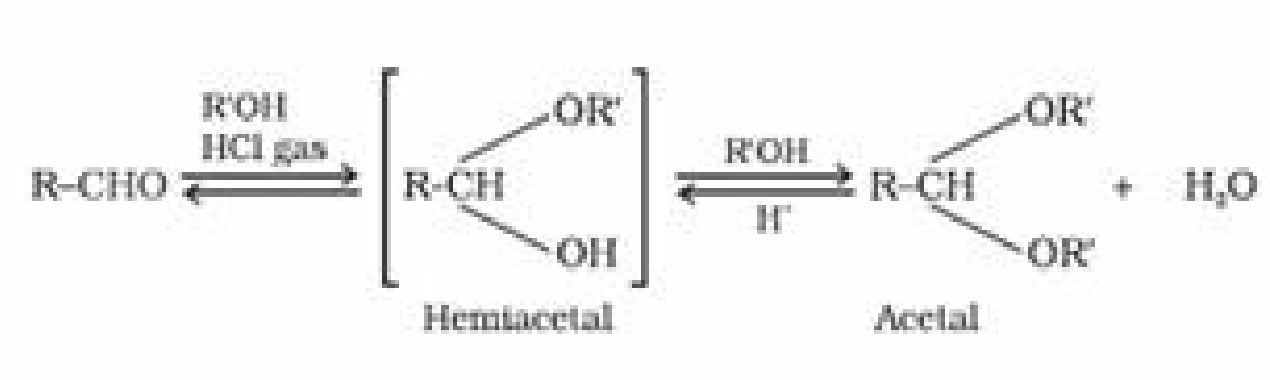
(d) Addition of alcohol:
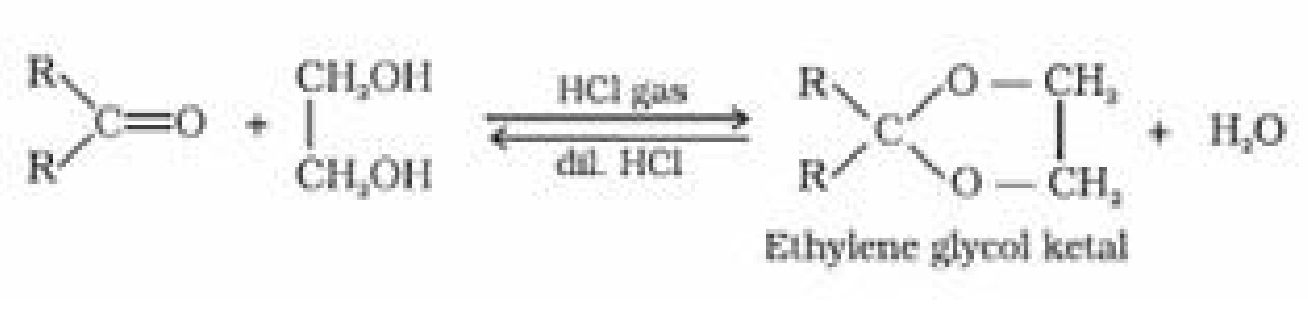
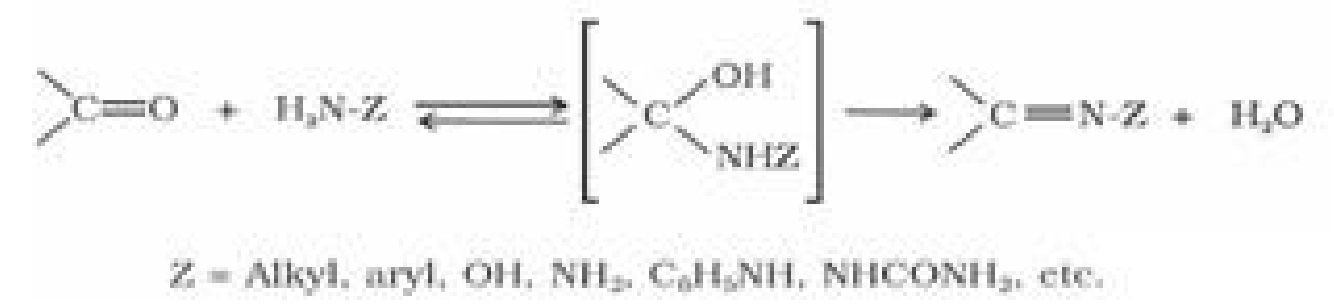
(e) Addition of ammonia and its derivatives:
Reduction of aldehydes and ketones:
(a) Reduction to alcohols:
Aldehydes and ketones on catalytic hydrogenation in presence of Ni, Pt or Pd by using
lithium aluminium hydride {LiAlH4) or sodium borohydride (NaBH4) forms primary
and secondary alcohols respectively.
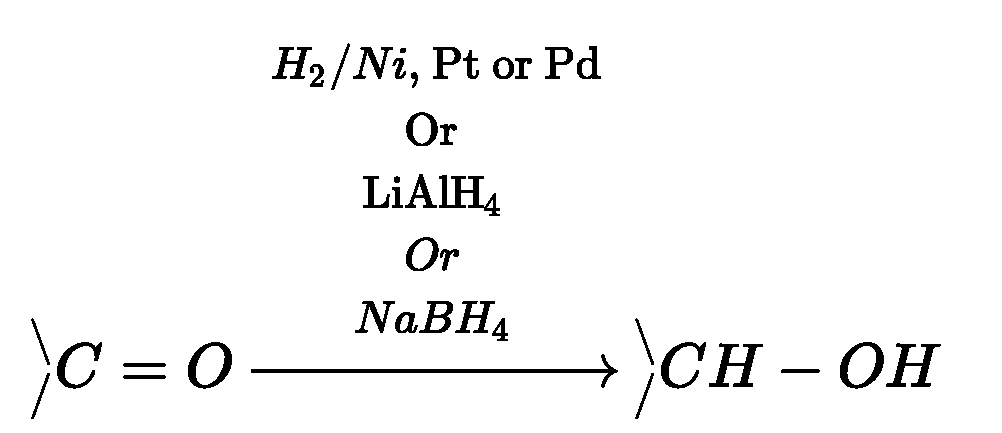
(b) Reduction to hydrocarbons:
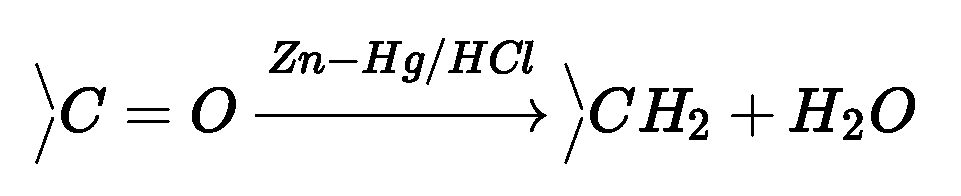
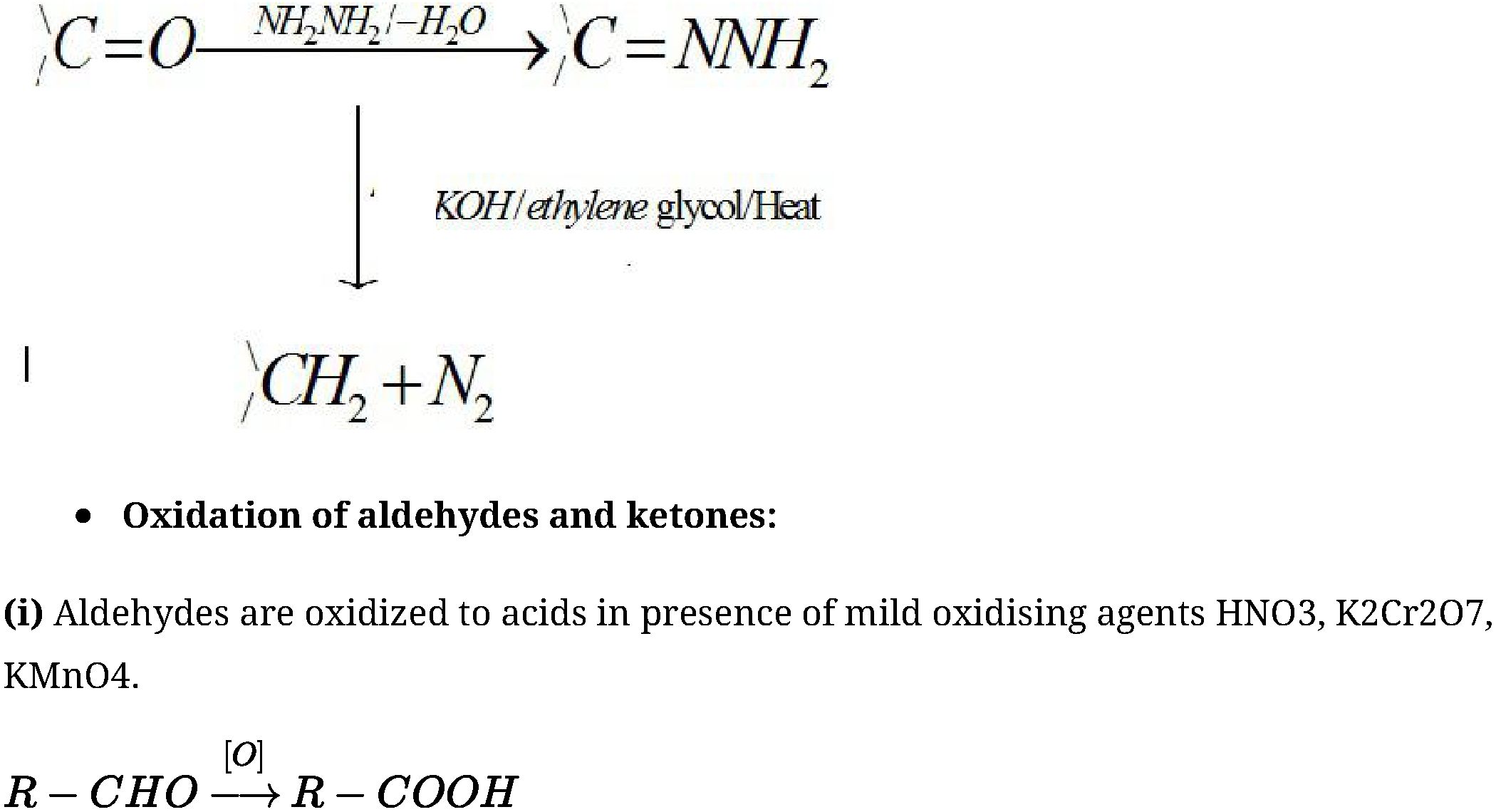
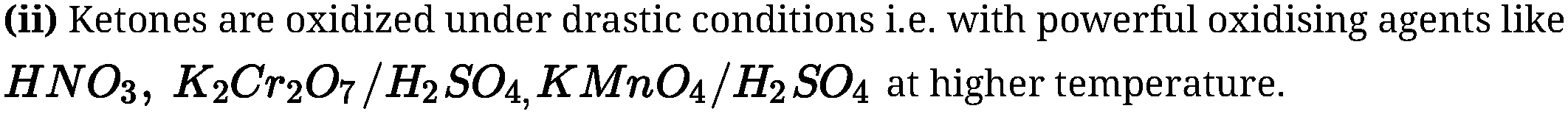
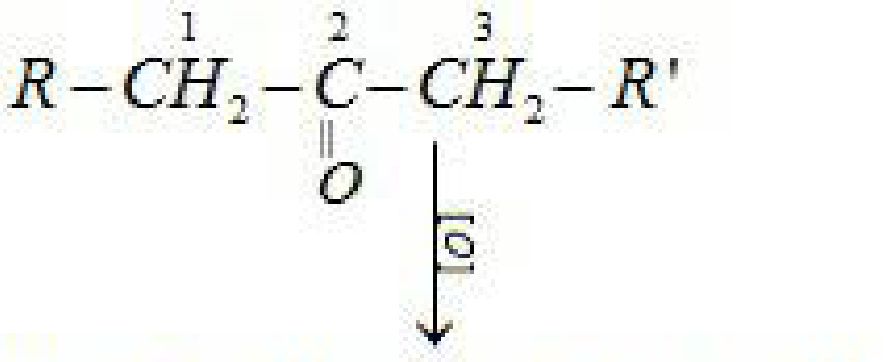
R-COOHH- J? -CHzCOOH
(By dfav^e OiCt-C^wif)
+
(j5T ■: lMV15* of Cj – Cjiw*J)
In case of unsymmetrical ketones cleavage occurs in such a way that keto group stays with
smaller alkyl group. This is known as Popoffs rule.
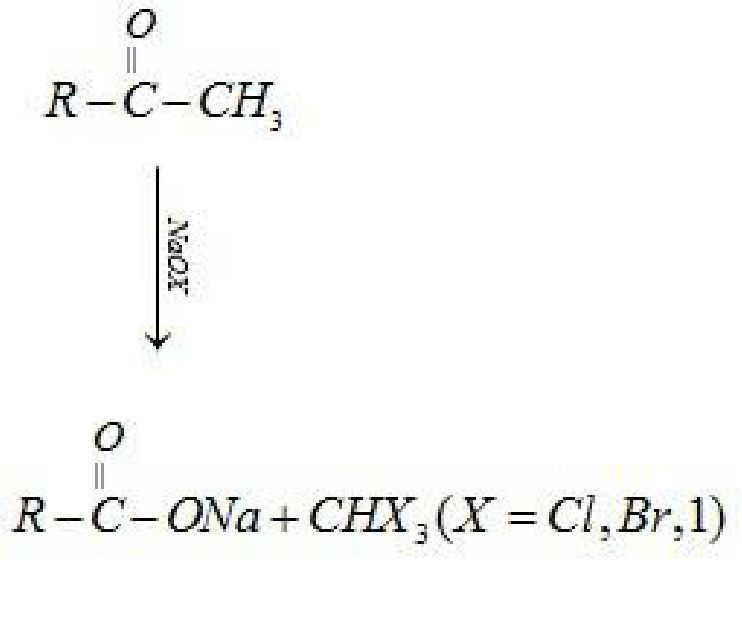
• Reactions of aldehydes and ketones due to a -hydrogen:
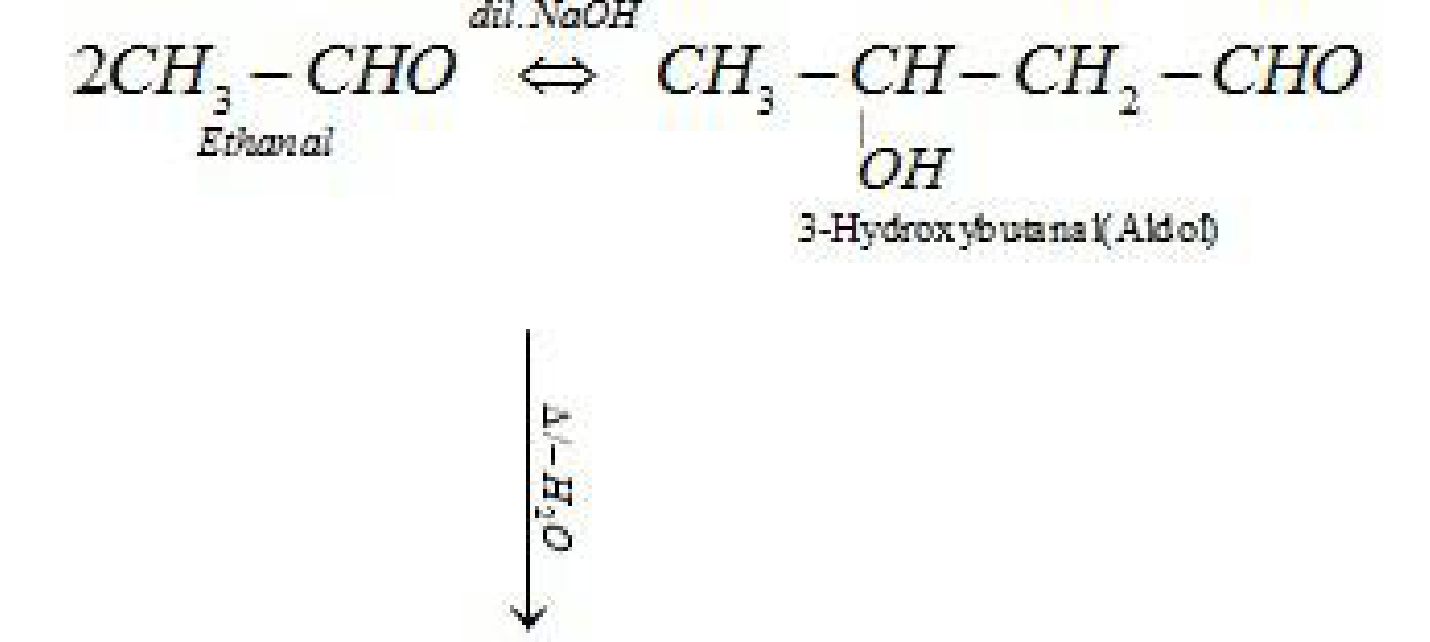
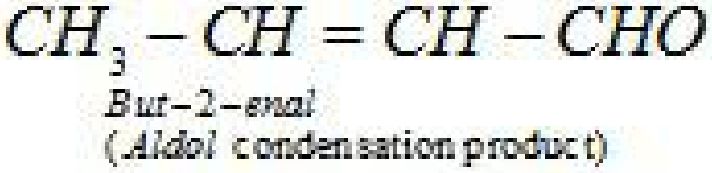
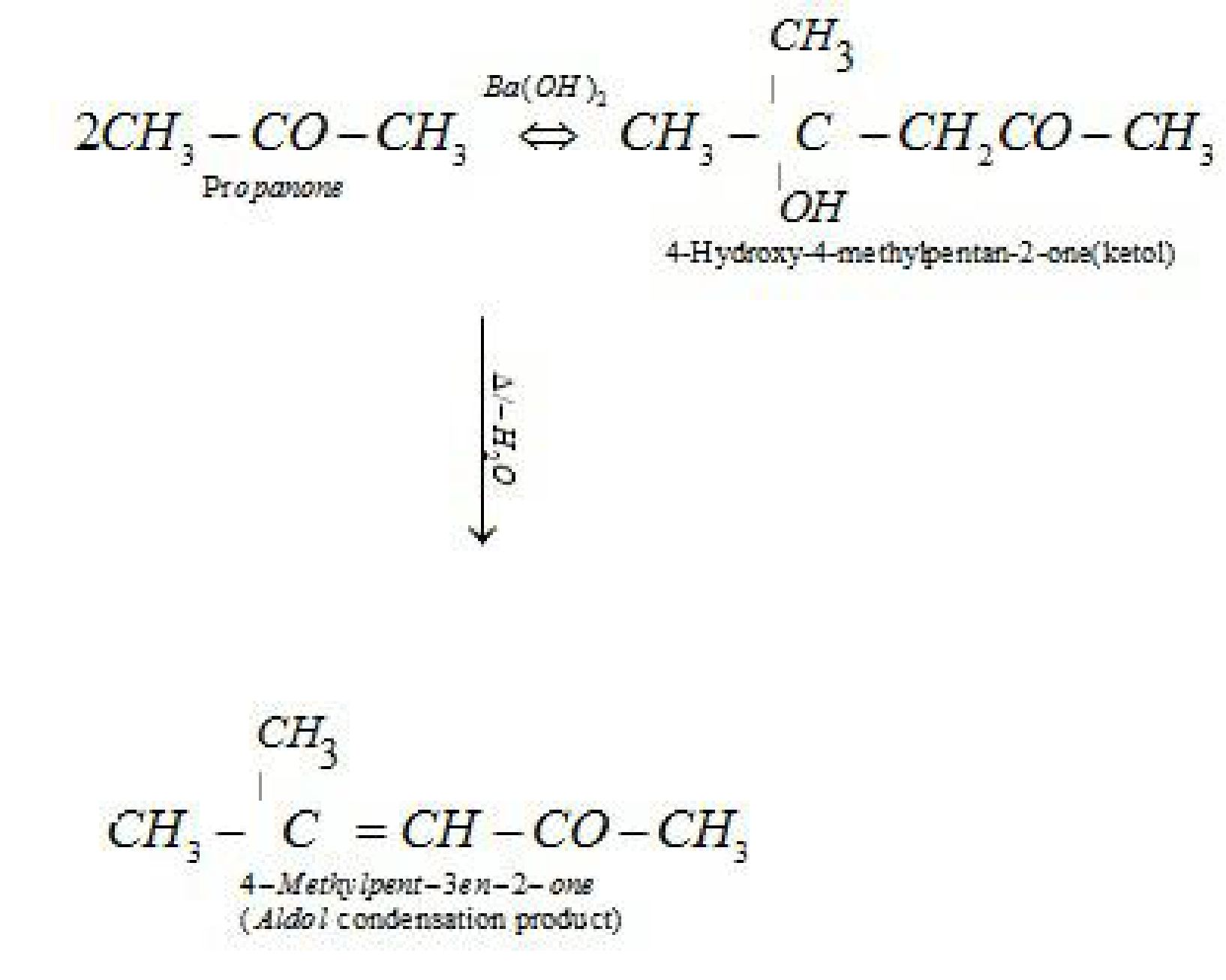
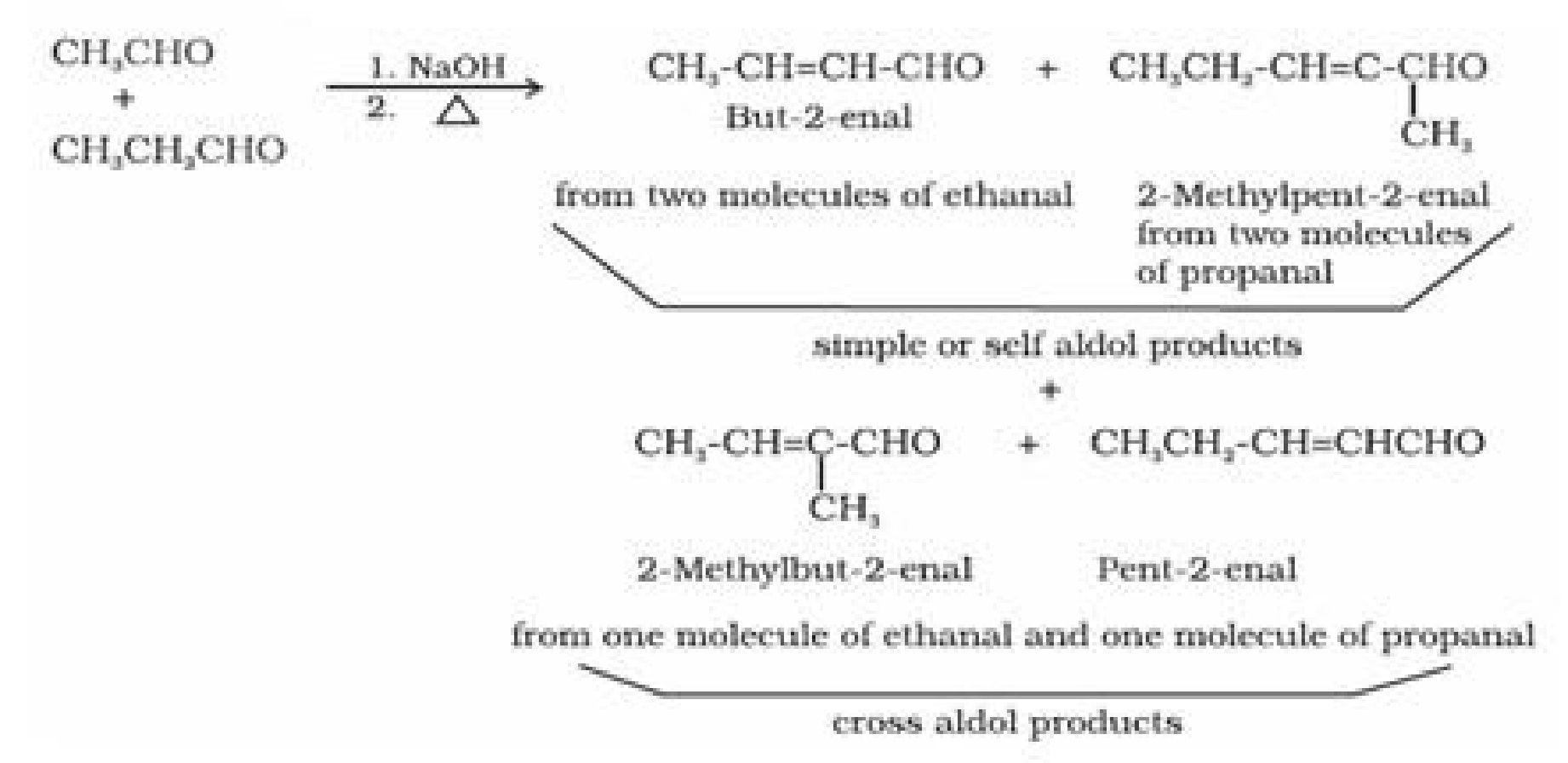
• Test to distinguish aldehydes and ketones:
Mi&+%j£m3)/+3ar ^Rcvai-24g+2H,o+4XH,
Ketones do not form silver mirror and hence do not give this test.
Kfc^-&n*n ppl
Ketones do not give this test.
• Carboxylic Acids:Carboxylic acids are the compounds containing the
carboxylfunctional group (-COOH).
0

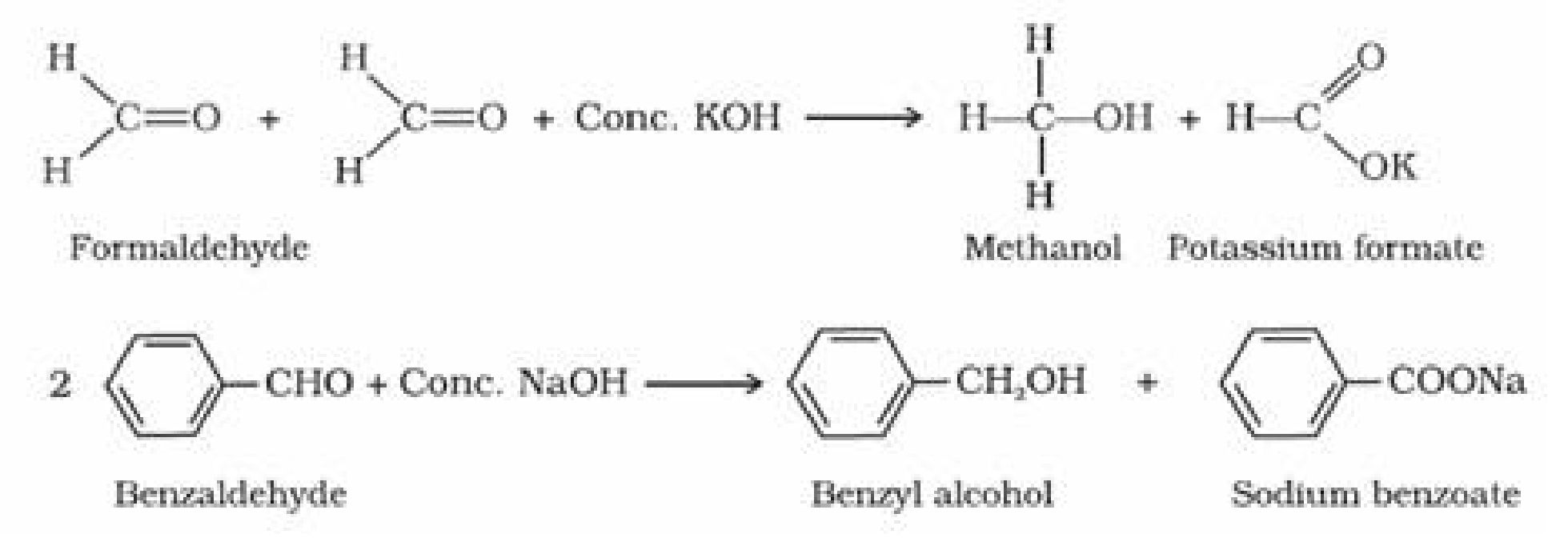
• Preparation of carboxylic acid:
(i) From alcohols: Primary alcohols are readily oxidised to carboxylic acids with common
oxidising agents such as potassium permanganate {KMnO4) in neutral, acidic or alkaline
media or by potassium dichromate (K2Cr2O7) and chromium trioxide (CrO3) in acidic
media.
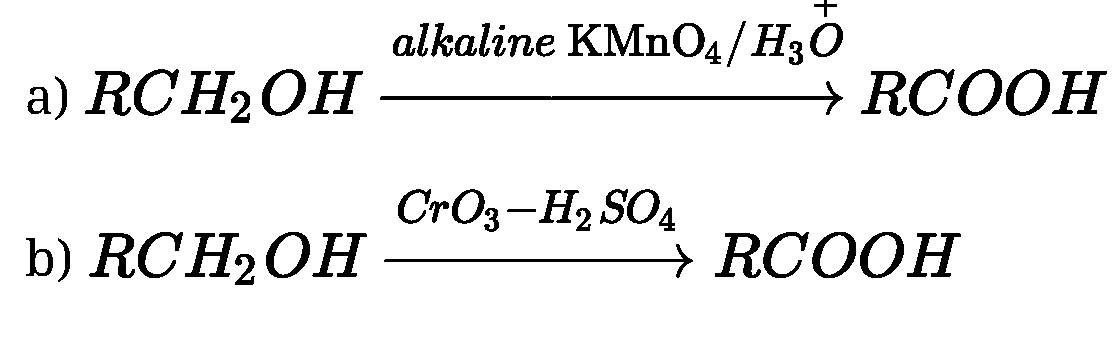
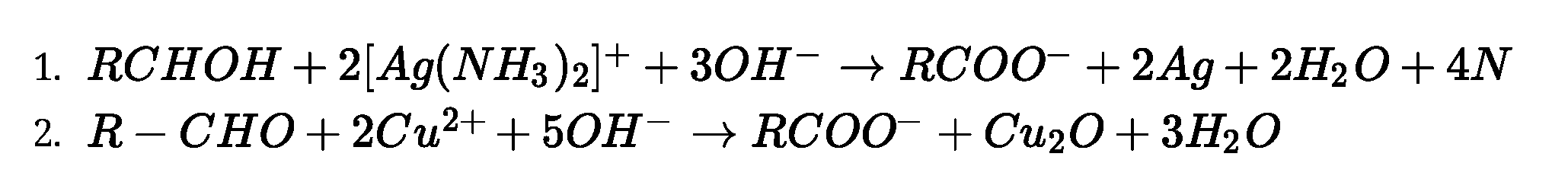

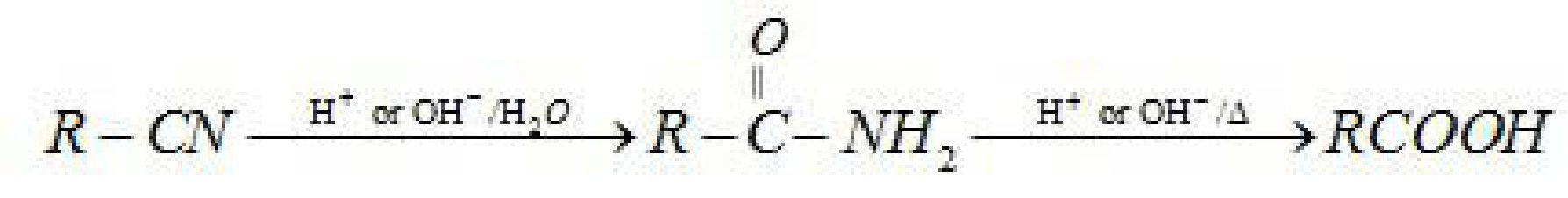
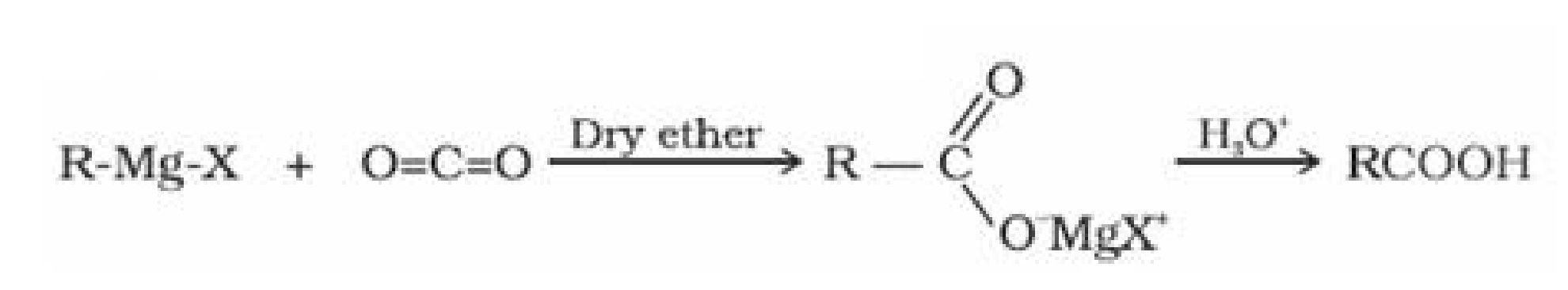
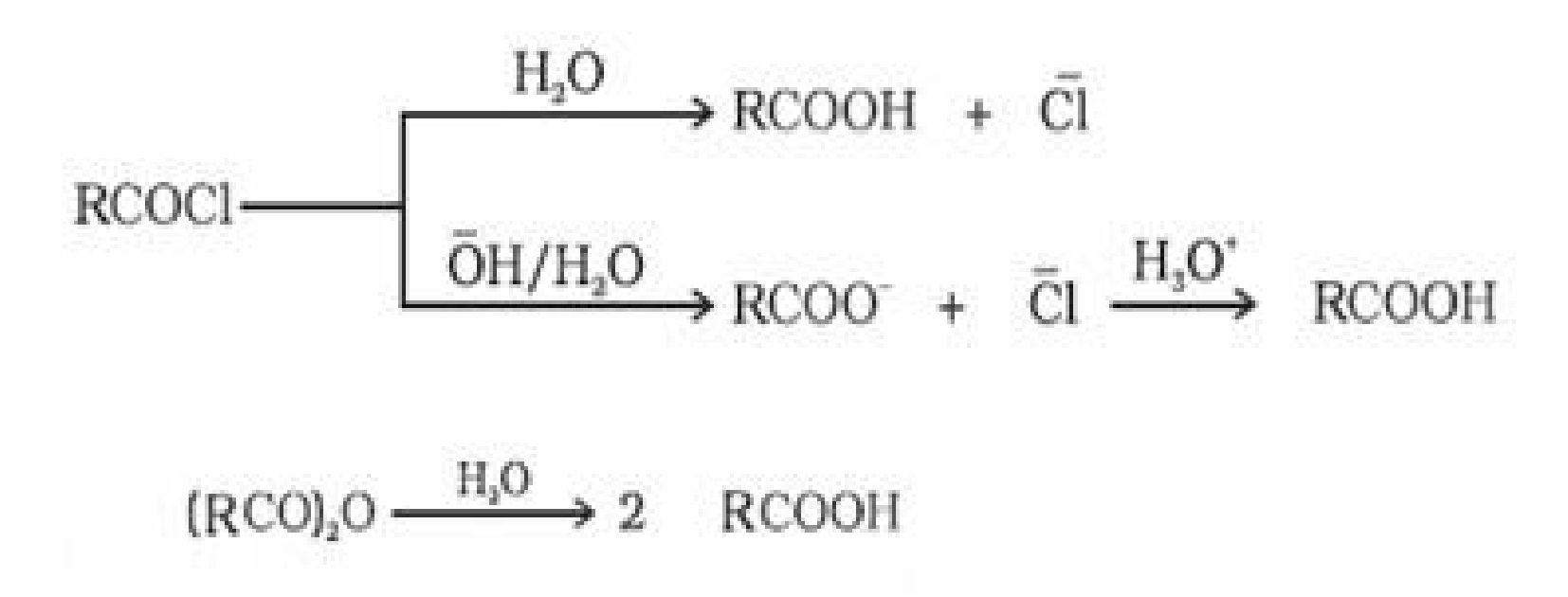
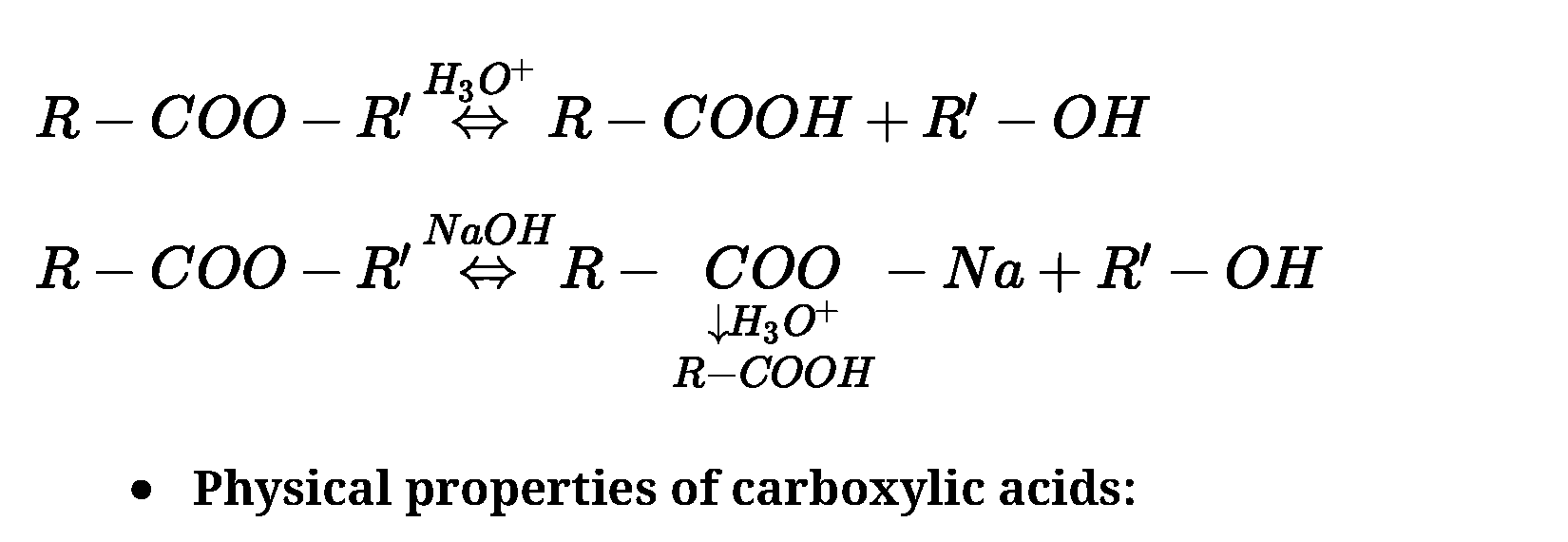
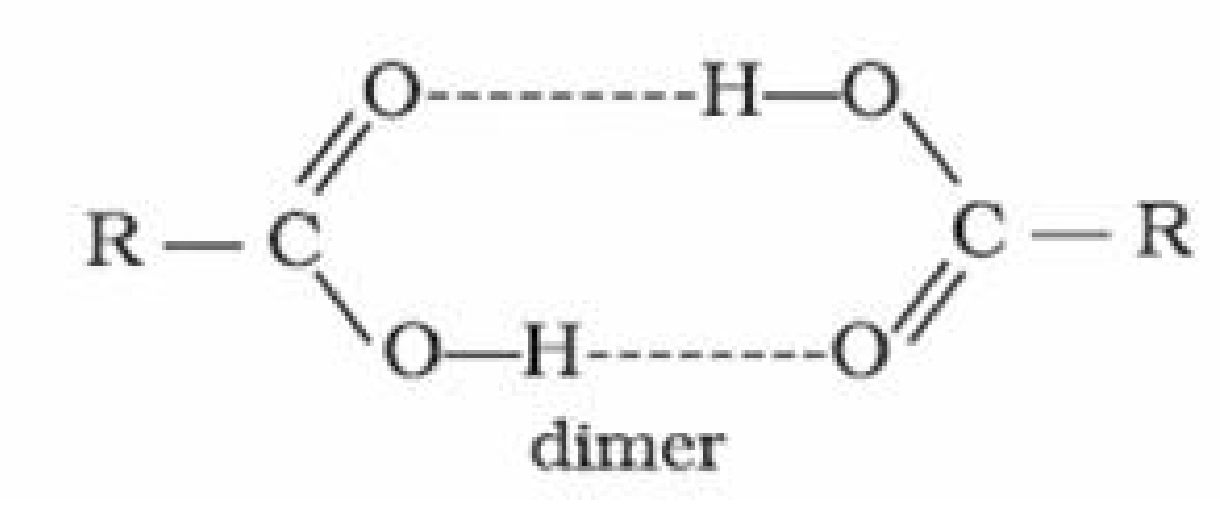
• Acidity of carboxylic acids:
Carboxylic acids are more acidic than phenols. The strength of acid depends on extent of
ionization which in turn depends on stability of anion formed.
• Reaction of carboxylic acids:
Reactions involving cleavage of C-OH bond:
Carboxylic acids on heating with mineral acids such as H2SO4 or with P2O5 give
corresponding anhydride.
(i) Anhydride formation:

(ii) Esterification: Carboxylic acids are esterified with alcohols in the presence of a mineral
acid such as concentrated H2SO4 or HCl gas as a catalyst.

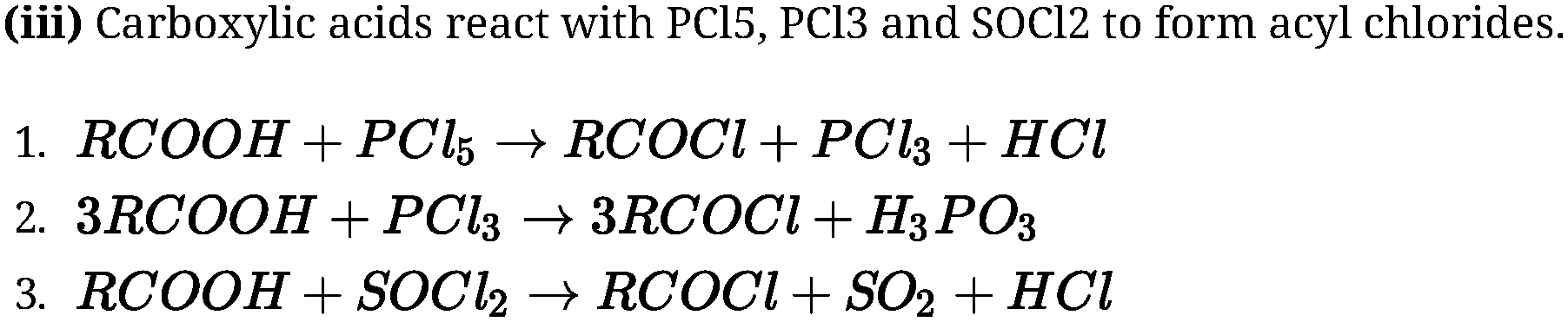
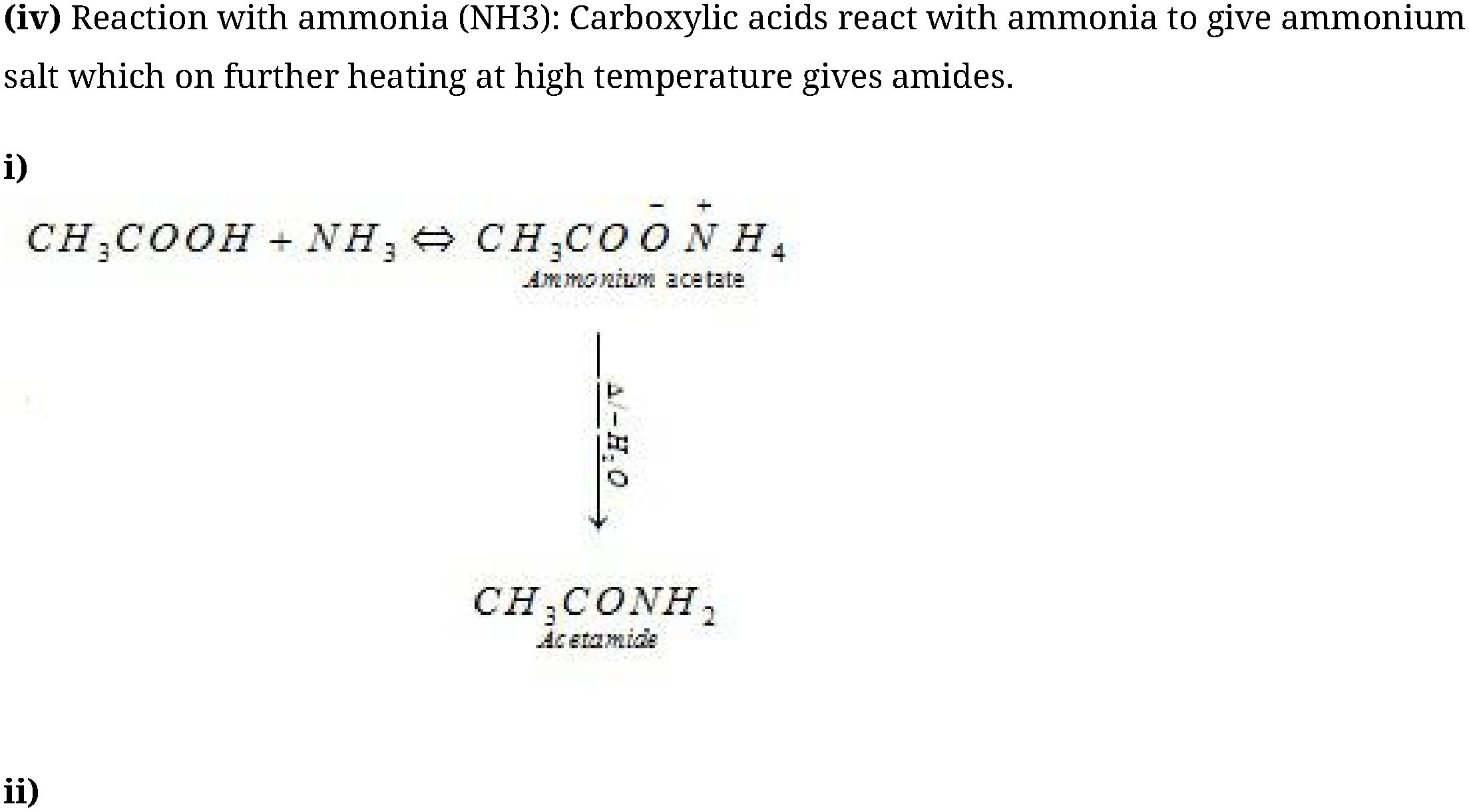

Auirnoniuin benzoatc Lfcnutmidc
Reactions involving COOH group:

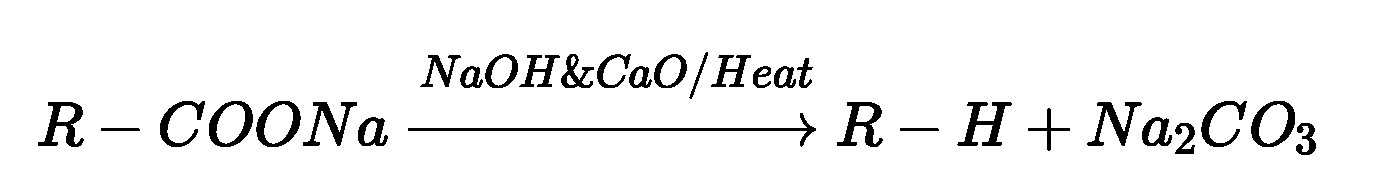
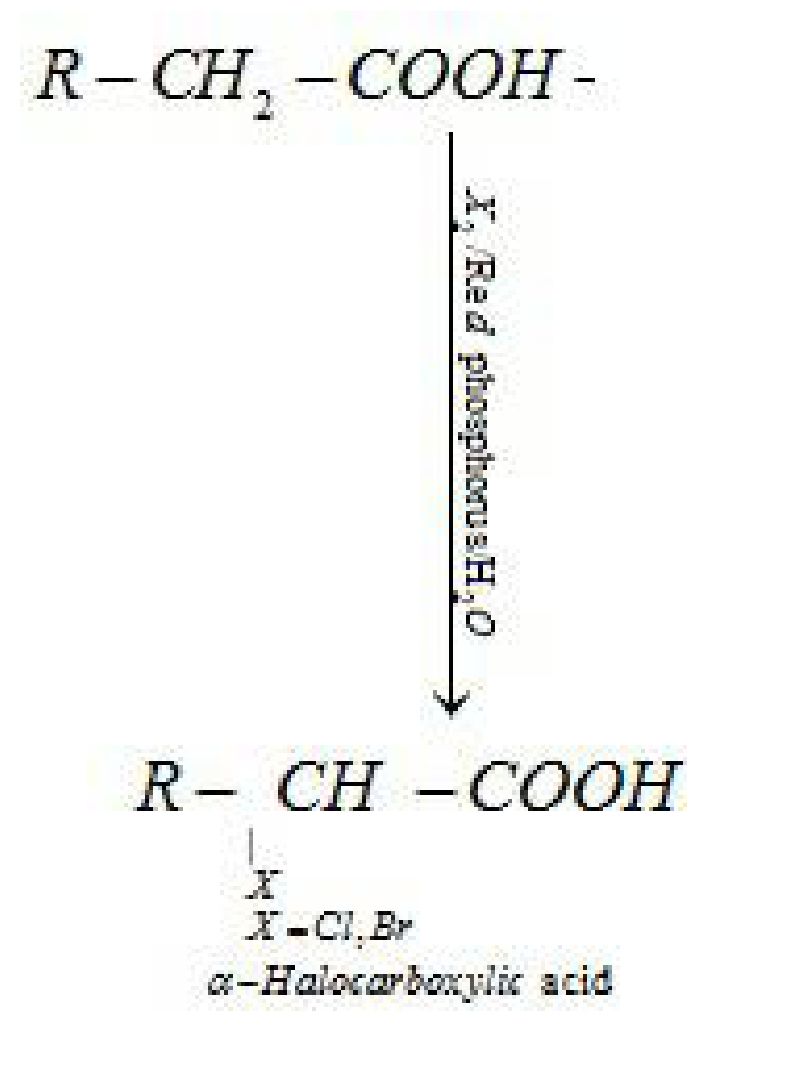
(i) Hell-Volhard-Zelinsky reaction: Carboxylic acids having an ct-hydrogen are halogenated
at the a-position on treatment with chlorine or bromine in the presence of small amount of
red phosphorus to give a-halocarboxylic acids)
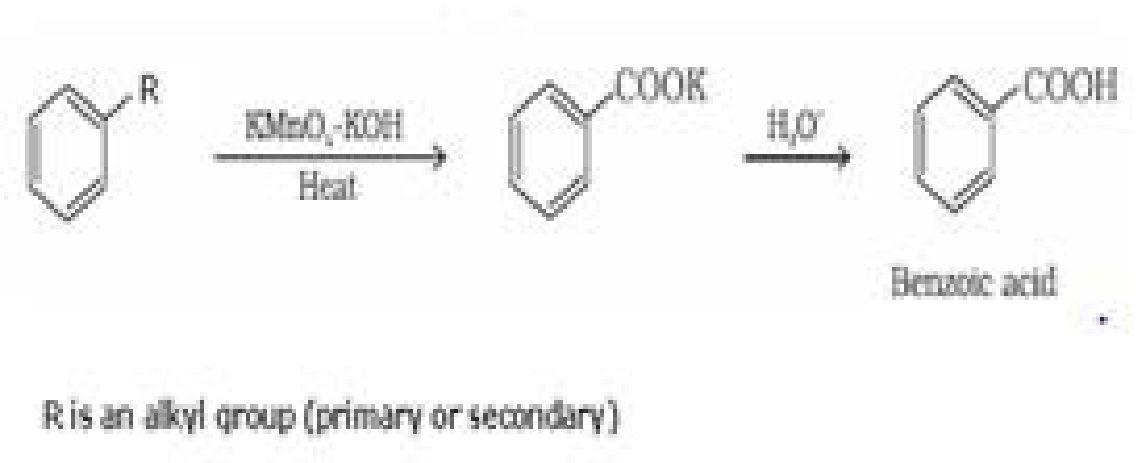
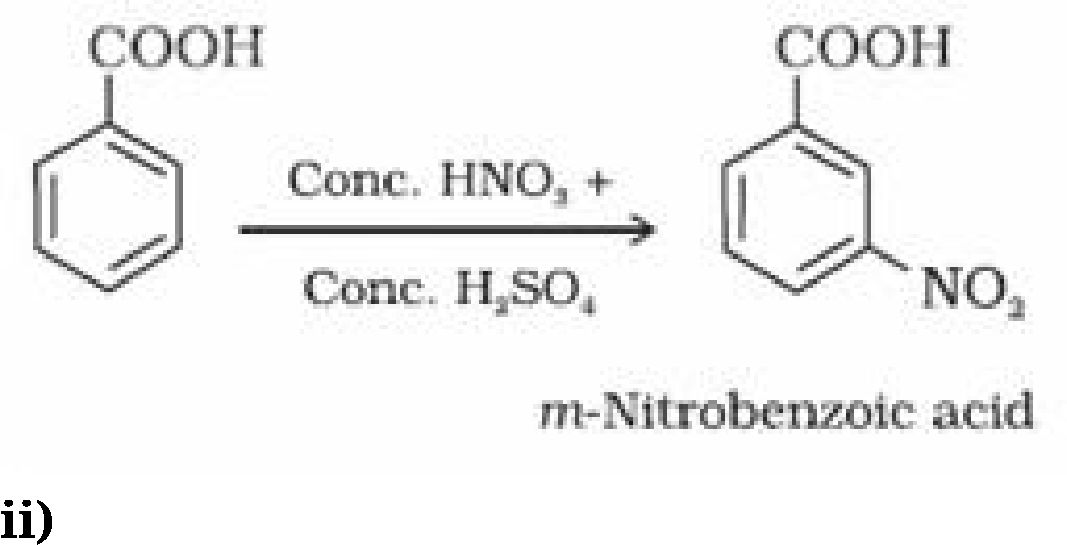
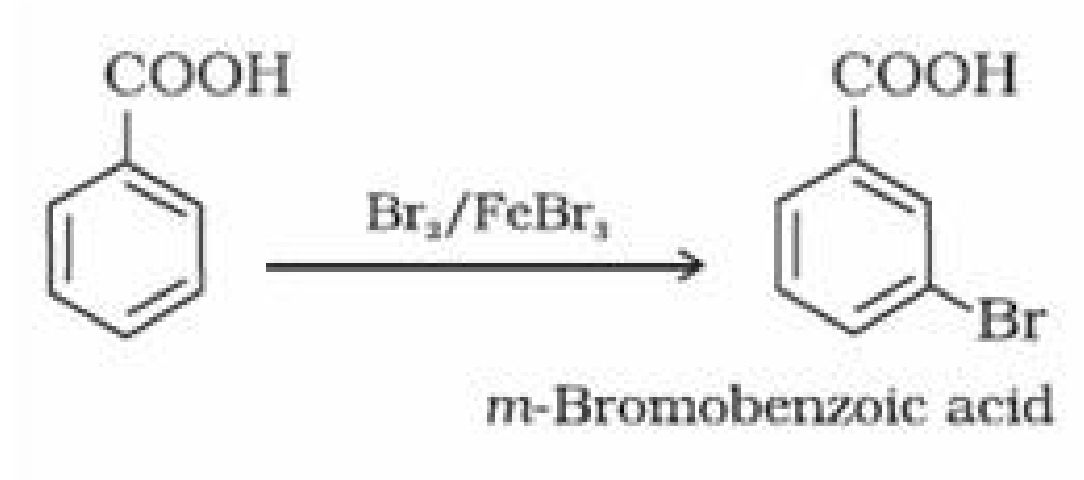
(ii) Ring substitution in aromatic acids: Aromatic carboxylic acids undergo electrophilic
substitution reactions. Carboxyl group in benzoic acid is electron withdrawing group and is
meta directing.
Canizzaro reaction: Aldehydes which do not have an a. -hydrogen atom undergo
self-oxidation and reduction (disproportionation) reaction on treatment with
concentrated alkali to form alcohol and salt of acid. ↑
CBSE Class 12 Chemistry
Quick Revision Notes
Chapter 11
Alcohols, Phenols and Ethers
MtQianol
(Akahd)
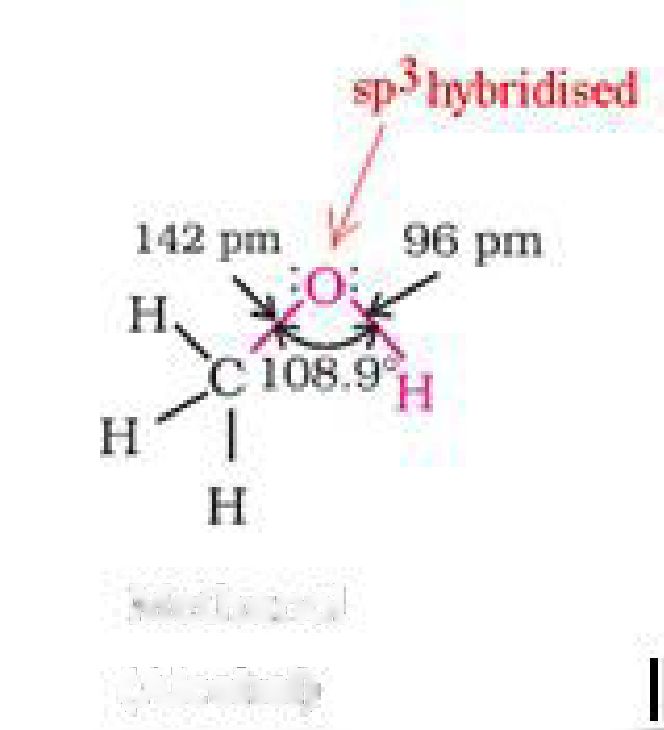
• Structure of alcohols:
Preparation of alcohols:
Acid catalysed hydration
(H 20,H+)
Mark, Addition
Or
Hydroboration – oxidation
B2H^H2O2IOH-
Mark, addition
Product is anti mark
Alkene > Alcohol
H2 catalyst
Esters > Alcohol
H2jPdor
NaBHi OrLiAlHi OrGrignard’s reagent
Aldehyde and ketone
Alcoho 1 <r
Alcoho 1 i Carboxylic acids
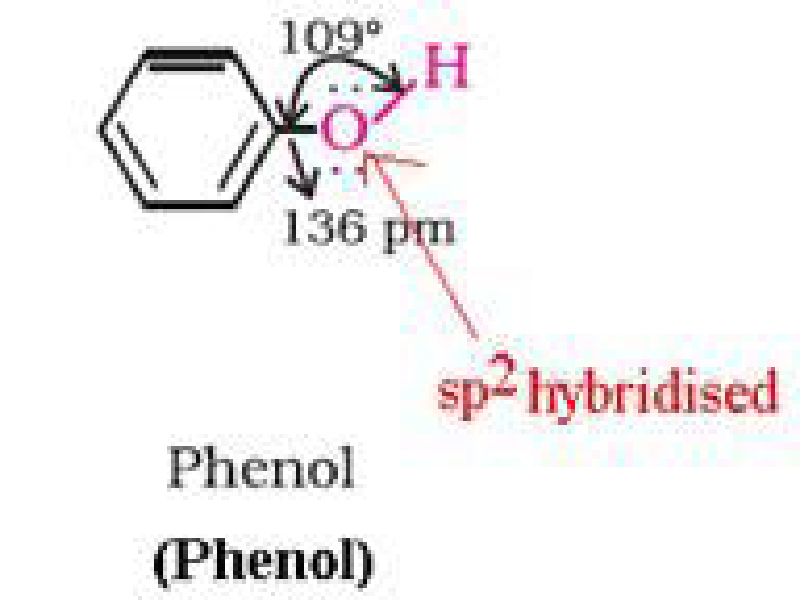
• Structure of phenols:
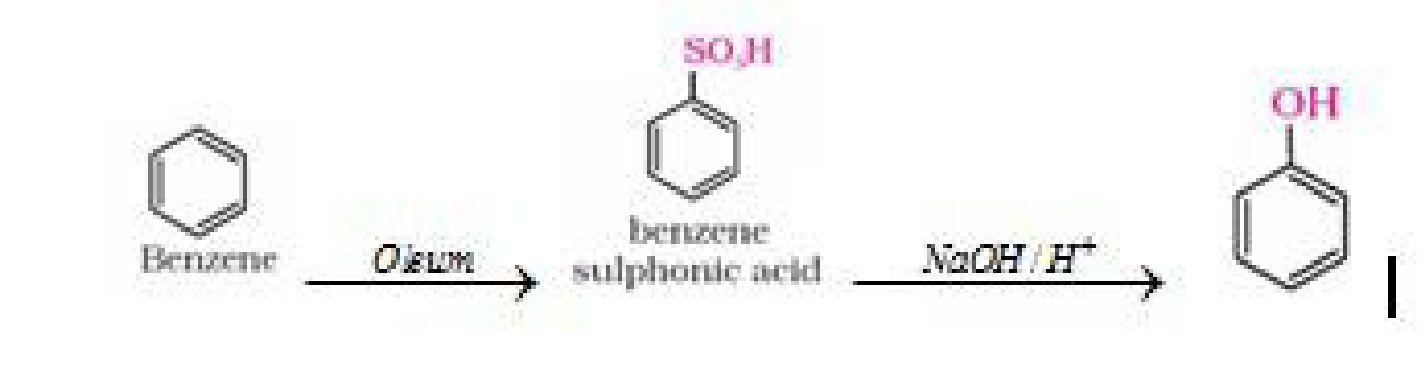
a) From benzene
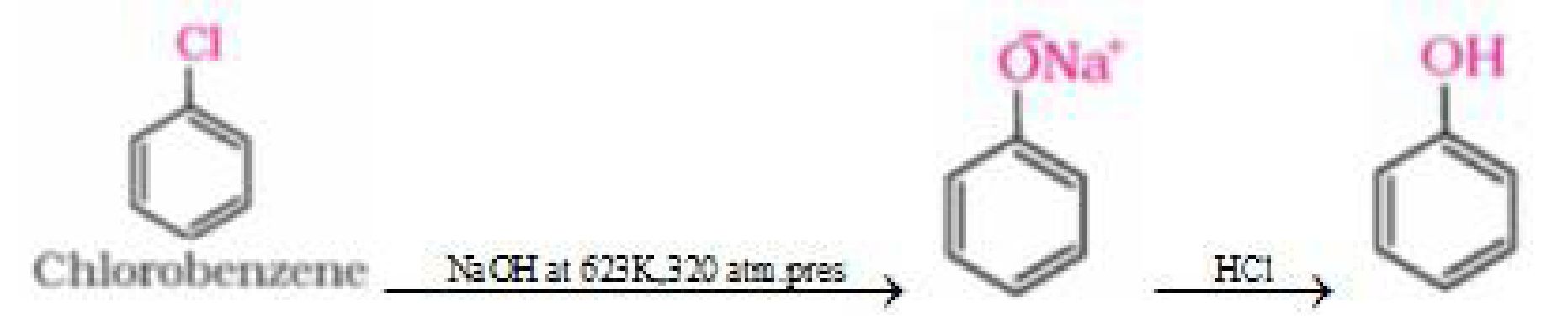
b) From chlorobenzene
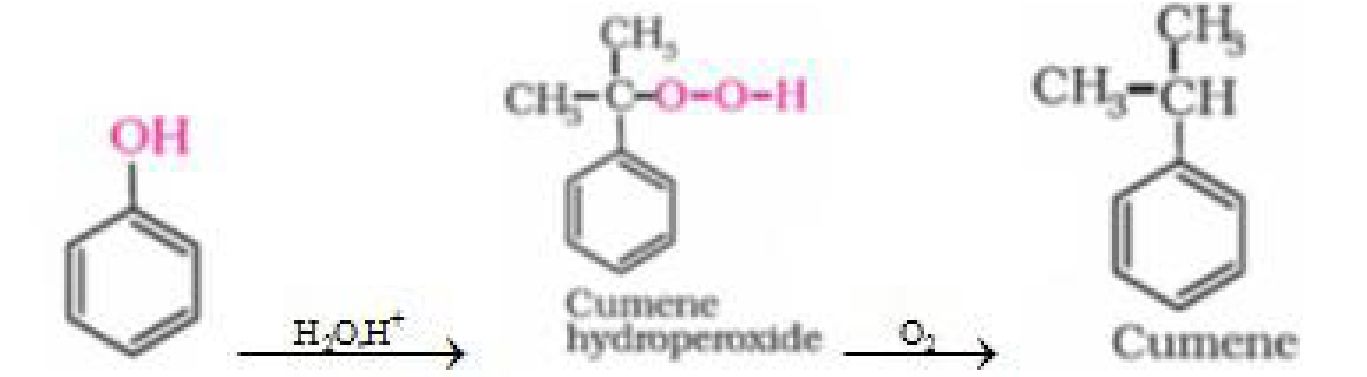
c) From cumene
d) From aniline
Physical properties of alcohols and phenols:
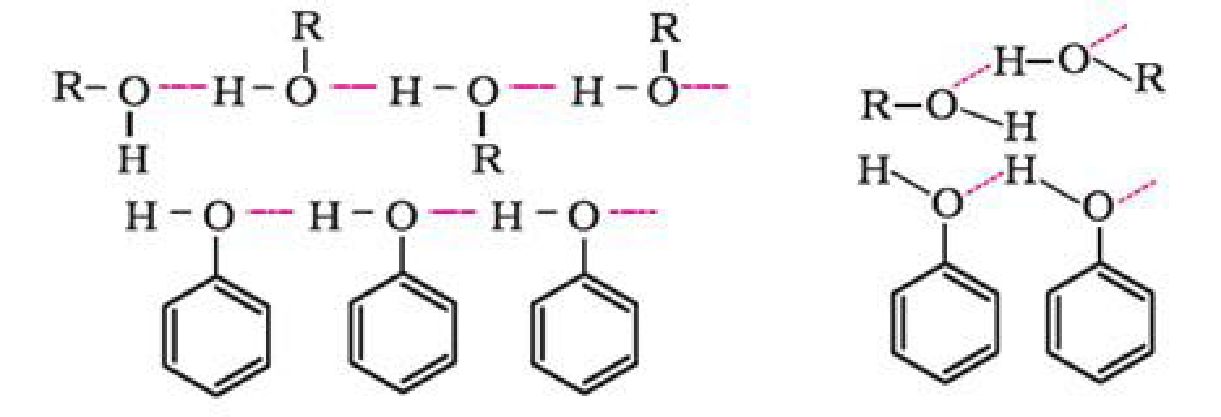
The boiling points of alcohols and phenols increase with increase in the number of carbon
atoms. This is because of increase in van der Waals forces with increase in surface area.
In alcohols, the boiling points decrease with increase of branching in carbon chain. This is
because of decrease in van der Waals forces with decrease in surface area.
• Chemical properties of alcohols:
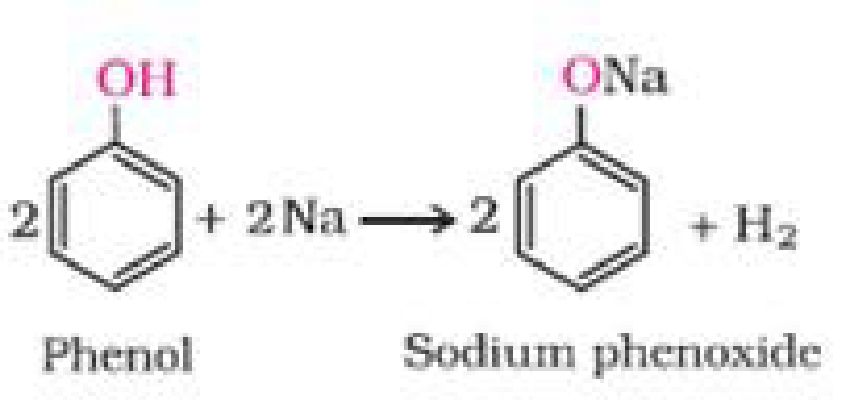
2 R – O – H + 2 Na ^ 2 R – O – Na + H2
Sodium alkoxide
RO – H + R’ – COOH S ROCOR’ + H2 O
Alcohol
RO – H + (R!C02)0%- ROCOR + RCOOH
Alcohol
Pnridinp
RO – H + R!COCl — ► R – OCORf + HCl
Alcohol
c0nc.HCl+ZnCl2/Lucas reagent
3ROH + PX3 ^ 3R – X + H3P03(X = Cl, Br)
P10tic&cids(conc.H2SO40rHzPO4)OrCatalysts(anhyd.ZnCl2 or alumina)
Acidified potassium permanganat
CU,573k
Or
CiOs
Or
PCC
CU,573k
Or
CrO3
ii)
CU,573k
Or
KMnOi
iii)
• Chemical properties of phenols:
I. Reactions involving cleavage of O-H bond: Alcohols react as nucleophiles:
a) Reaction with metals
b) Esterification reaction
Ar – OH + H – COOH ^> Ar – OCOR + H2O
Phenol
Ar – OH + (RCO)2O «■ Ar – OCOR + RCOOH
Phenol
P? m*n m 77 p
Ar – OiJ + RCOCl — > ArOCOR1 + iJC7
Phenol
II. Other chemical reactions of phenols:
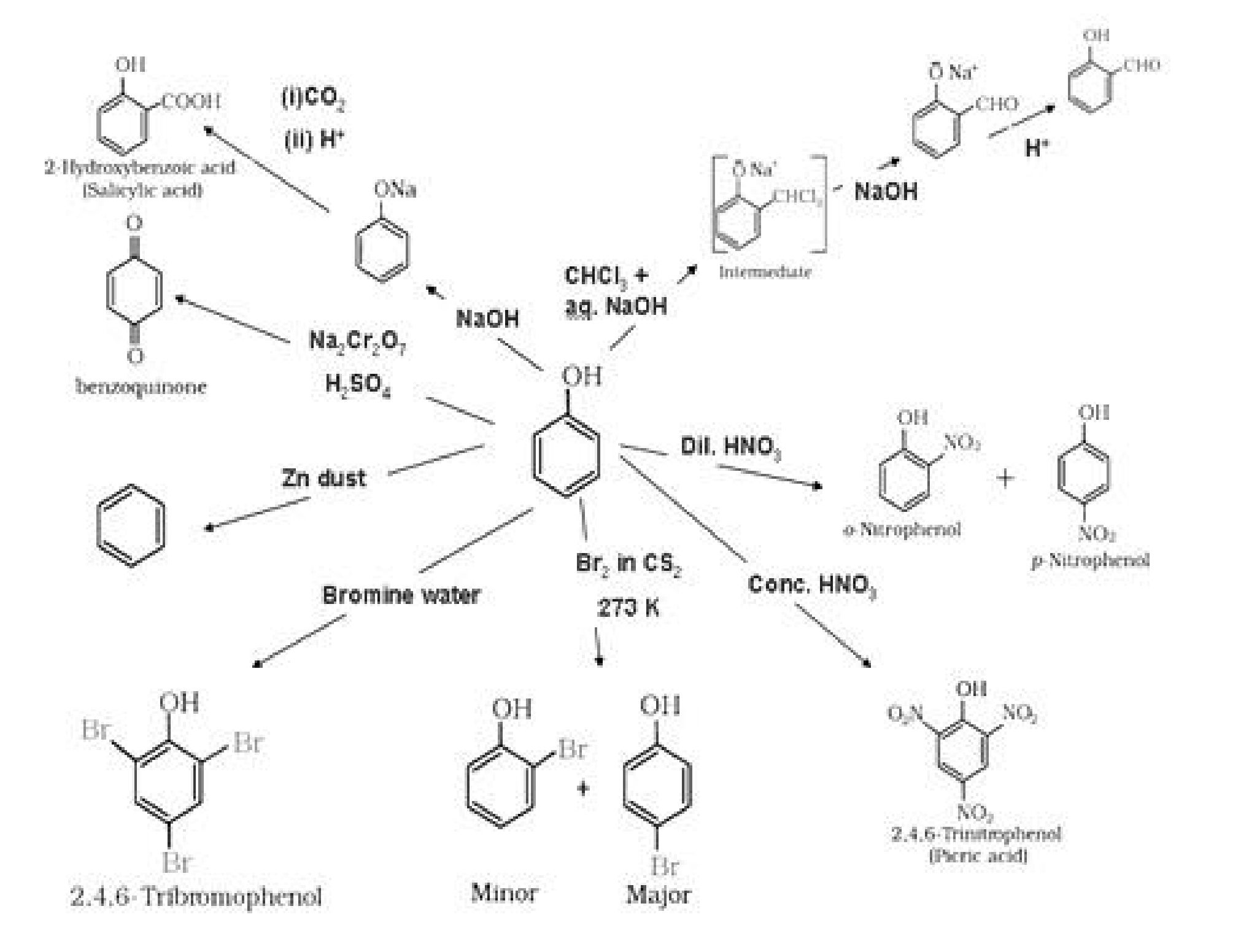
a). Phenol > H2O > Primary alcohol > Secondary alcohol > Tertiary alcohol.
The acidic character of alcohols is due to the polar nature of O-H bond. Alkyl group is an
electron-releasing group (-CH3, -C2H5) or it has electron releasing inductive effect (+I effect).
Due to +I effect of alkyl groups, the electron density on oxygen increases. This decreases the
/10
Material Downloaded From SUPERCOP
polarity of O-H bond. And hence the acid strength decreases.
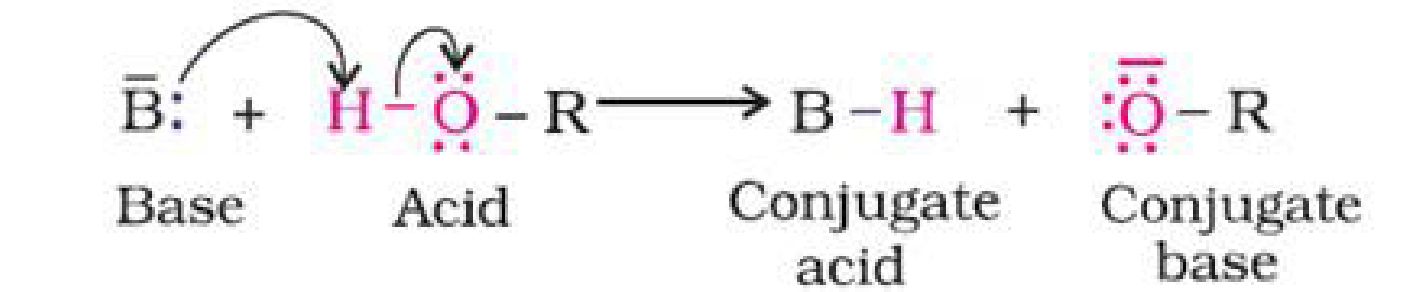
b) Phenol is more acidic than alcohol: In phenol, the hydroxyl group is directly attached to
the sp2hybridised carbon of benzene ring which acts as an electron withdrawing group
whereas in alcohols, the hydroxyl group is attached to the alkyl group which have electron
releasing inductive effect. In phenol, the hydroxyl group is directly attached to the
sp2hybridised carbon of benzene ring whereas in alcohols, the hydroxyl group is attached to
the sp3hybridised carbon of the alkyl group. The sp2hybridised carbon has higher
electronegativity than sp3hybridised carbon. Thus, the polarity of O-H bond of phenols is
higher than those of alcohols. Hence, the ionisation of phenols is higher than that of alcohols.
The ionisation of an alcohol and a phenol takes place as follows:
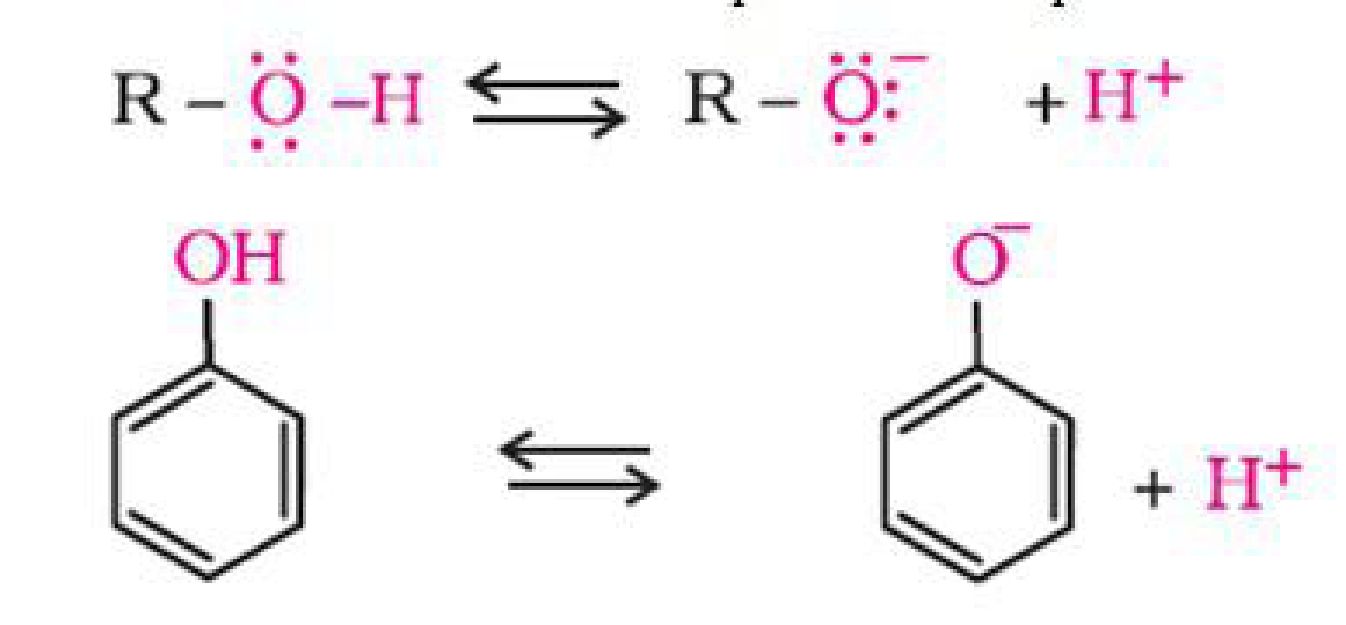
In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ion, the charge
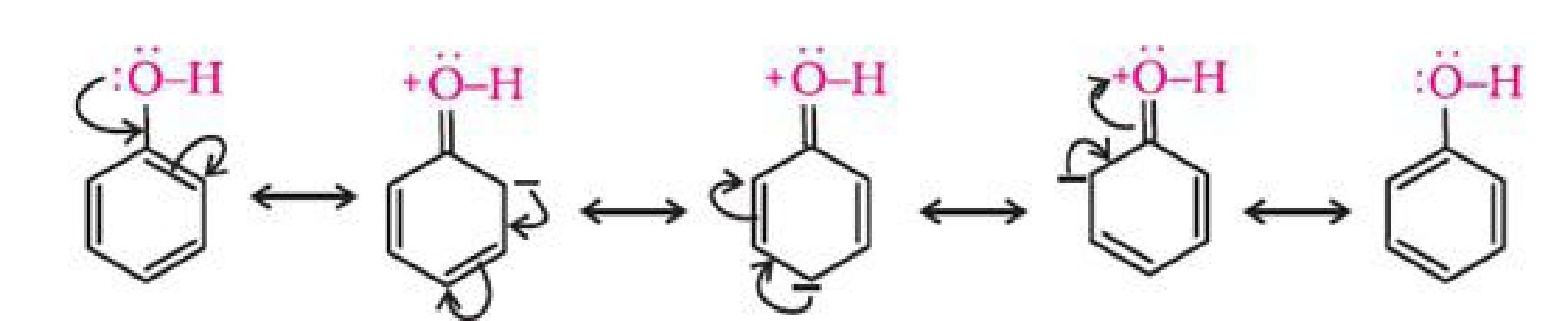
is delocalised.
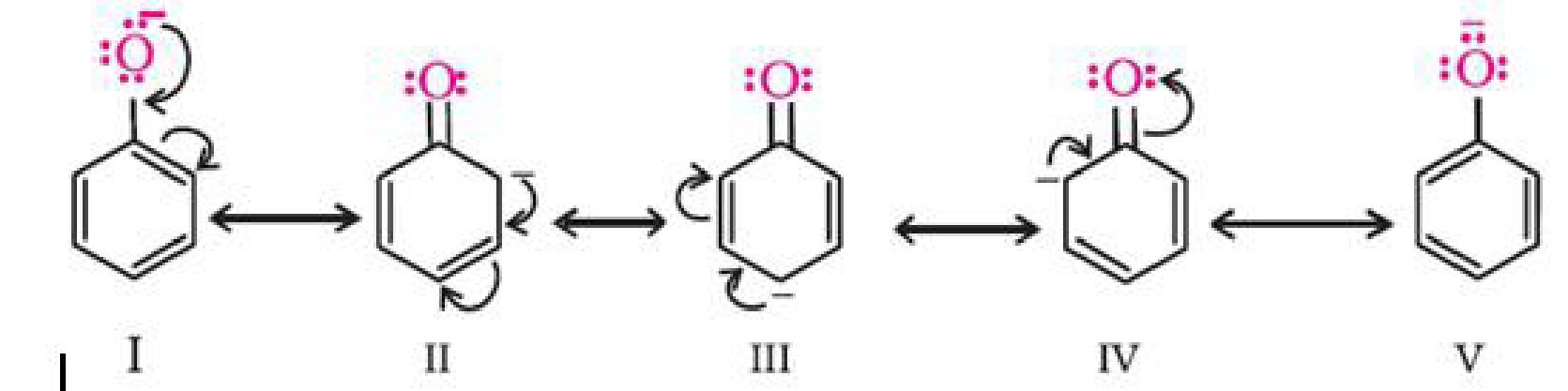
The delocalisation of negative charge makes phenoxide ion more stable and favours the
ionisation of phenol. Although there is also charge delocalisation in phenol, its resonance
structures have charge separation due to which the phenol molecule is less stable than
phenoxide ion.
• Differentiate between organic compounds:
Phenol on reaction with neutral FeCl3 gives purple colour whereas alcohols do not give
purple colour.
QCiH5OH + Fe3+ ^ [Fe(OCiH5)6}3– + 6
Purple colour
c0nc.HCl+ZnCl2/Lucas reagent
If it is a primary alcohol, no turbidity appears at room temperature. Turbidity appears only
on heating. If it is a secondary alcohol, turbidity appears in 5 minutes. If it is a tertiary
alcohol, turbidity appears immediately.
Iodoform test: Ethanol when reacted with (I2 and NaOH) or NaOI gives yellow ppt of
iodoform since it has the presence of CH3-CH (OH)- group.
C2H5OH + 4J2 + WaOH^ CHI3 + hNaI + §H20 + HC00Na
Yellow ppt
CH3OH +12 + NaOH ^ No yellow ppt
sp^hytmdffied
i
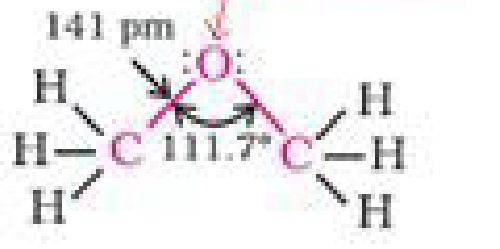
Msthoatymfihajif
0fttec)
• Preparation of ethers:
a) From alcohols
H2SO4 or H3PO4 at413K
Alcohol > Ethers
b) From alkyl halide and sodium alkoxide
Williamson! s synthesis
Ethers < Alkyl halide and sodium alkoxide
Here, the alkyl halide should be primary and alkoxide should be tertiary. In case of aromatic
ether, the aromatic part should be with phenoxide ion.
• Physical properties of ethers:
a) Miscibility: Miscibility of ethers with water resembles those of alcohols of the same
molecular mass. This is due to the fact that just like alcohols, oxygen of ether can also form
hydrogen bonds with water molecule.
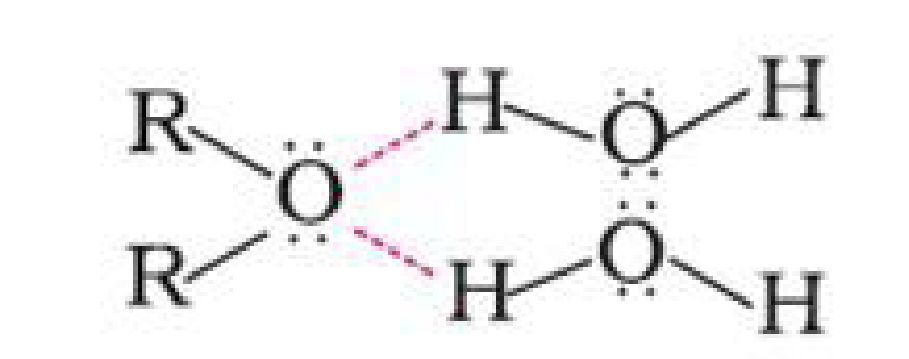
Ethers have much lower boiling points than alcohols. This is due to the presence of hydrogen
bonding in alcohols. Hydrogen bonding is absent in ethers.
R R
1. J
l i
j H *
Chemical properties of ethers:
a) Cleavage of C-O bond in ethers:
R-O-R’ + HX ^ R-X + R’OH
Excess
The order of reactivity of hydrogen halides is as follows: HI >HBr>HCl
Alkyl halide formed is always the lower alkyl group. But if a tertiary alkyl group is present,
the alkyl halide is always tertiary. In case of phenolic ethers, the cleavage occurs with the
formation of phenol and alkyl halide.
b) Electrophilic substitution reaction in aromatic ethers:
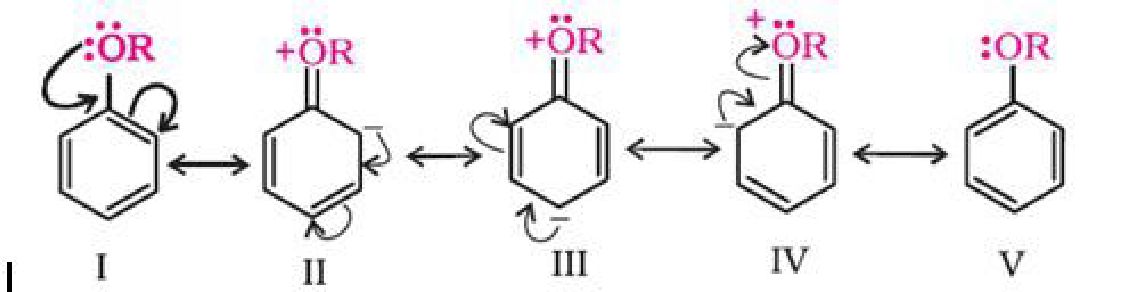
The electrophilic substitution reaction of aromatic ether involves the following reaction:
• Other conversion reactions:
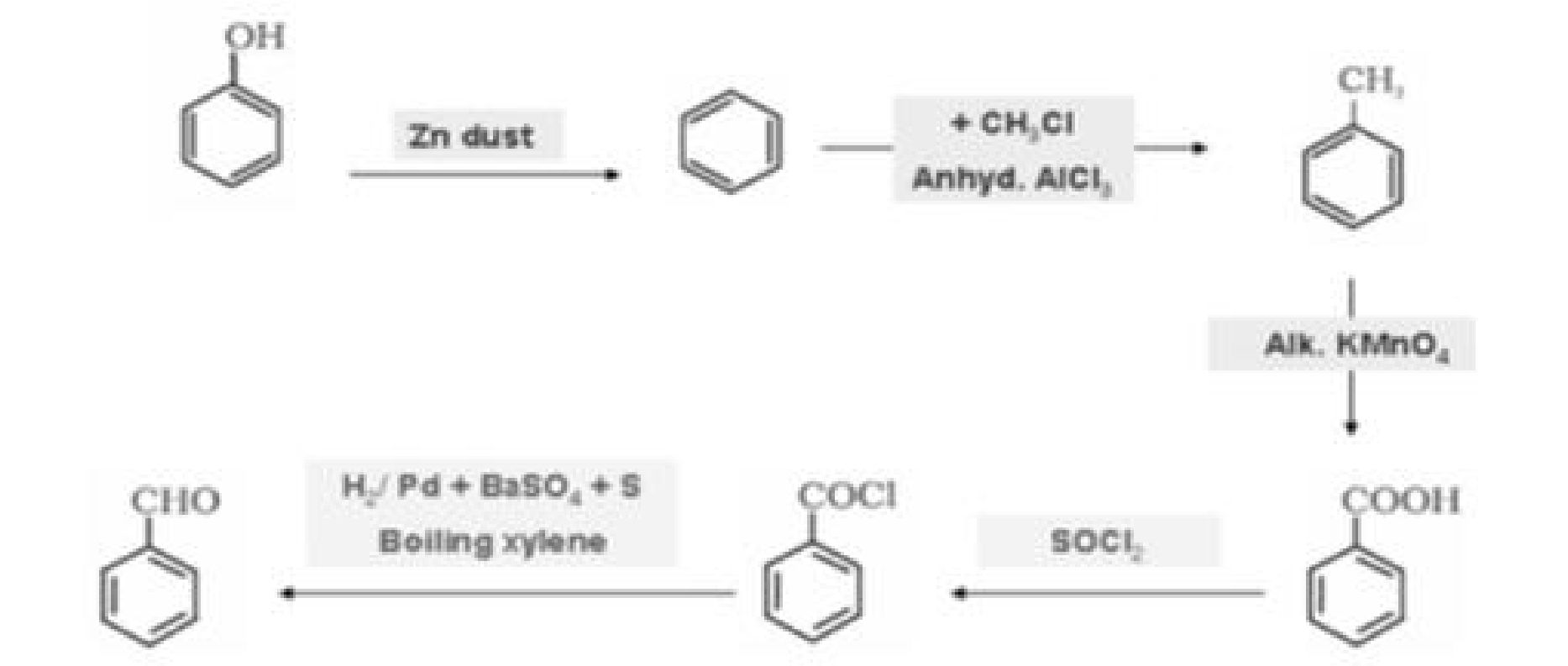
a) Phenol to salicyldehyde
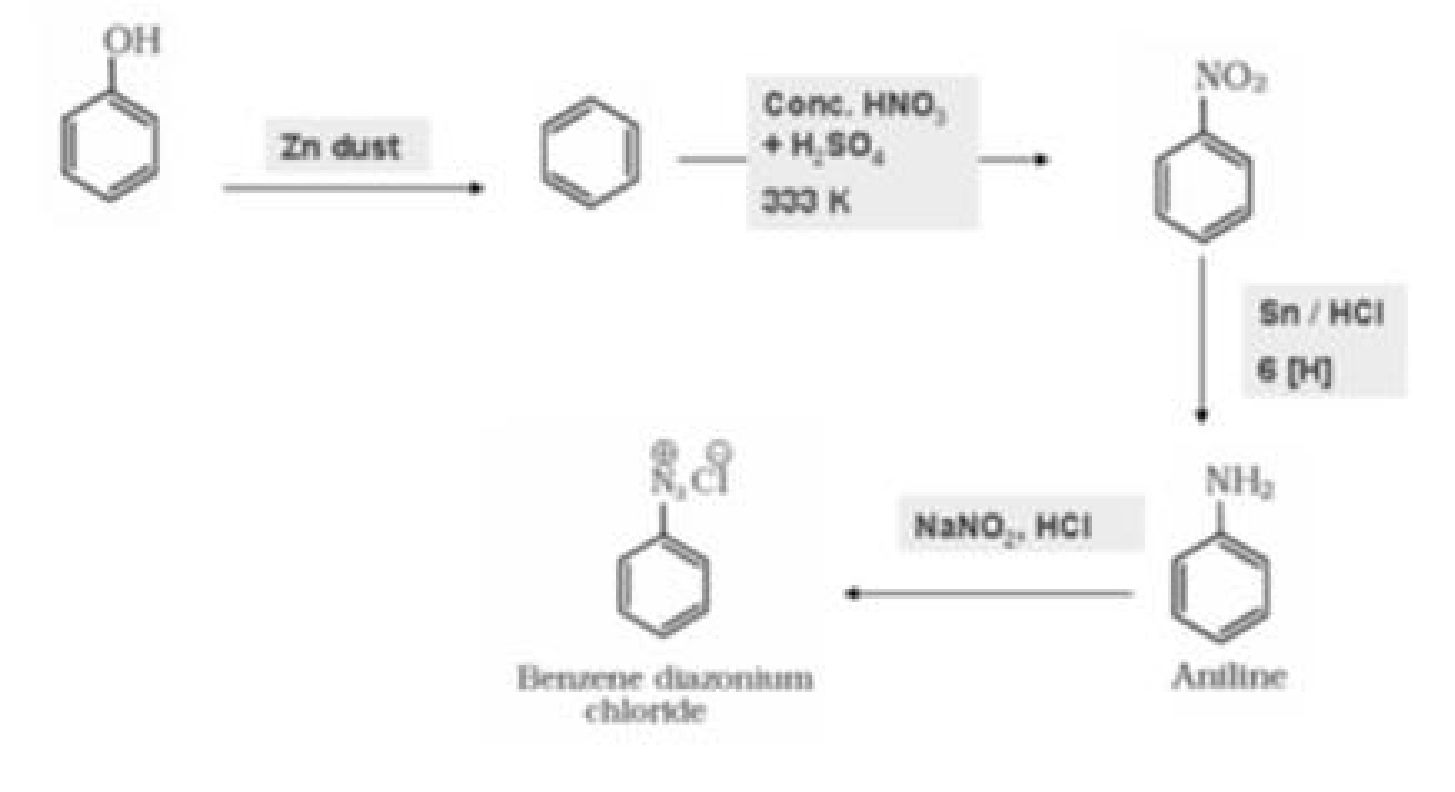
b) Phenol to benzene diazonium chloride
CBSE Class 12 Chemistry
Quick Revision Notes
Chapter 10
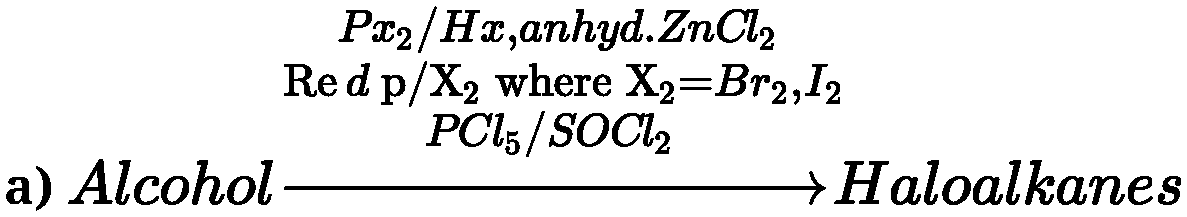



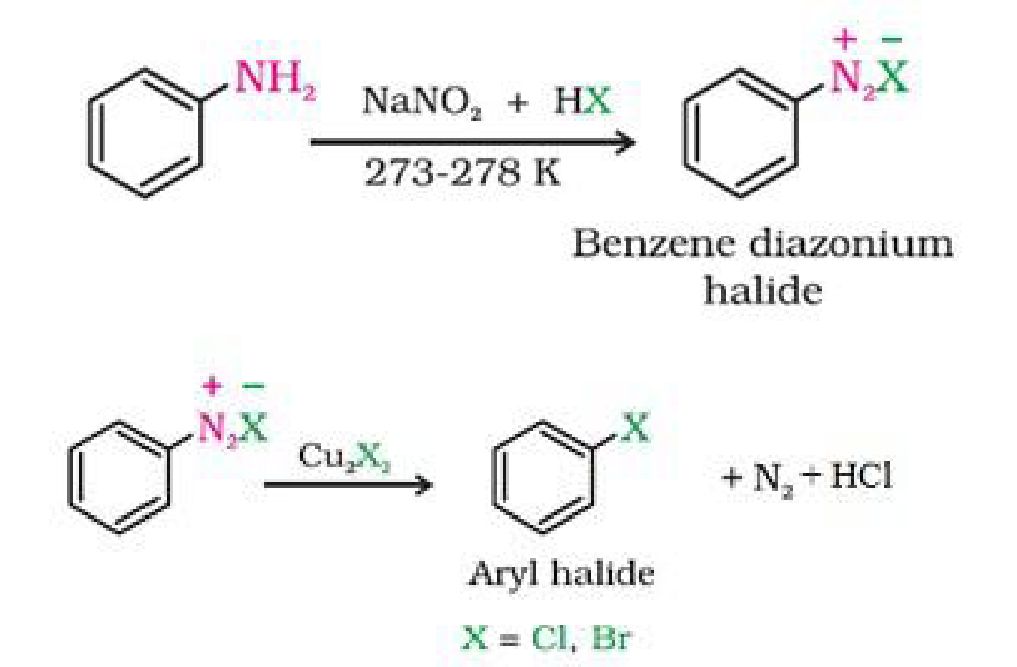
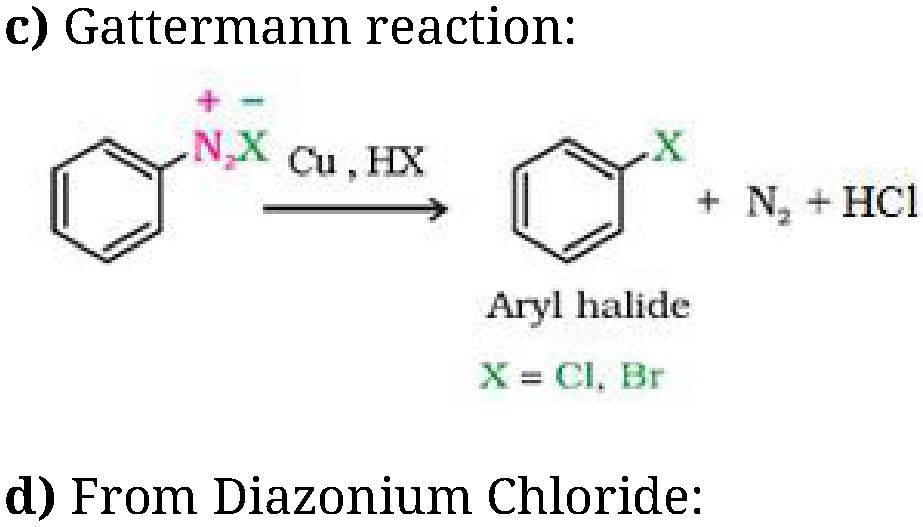
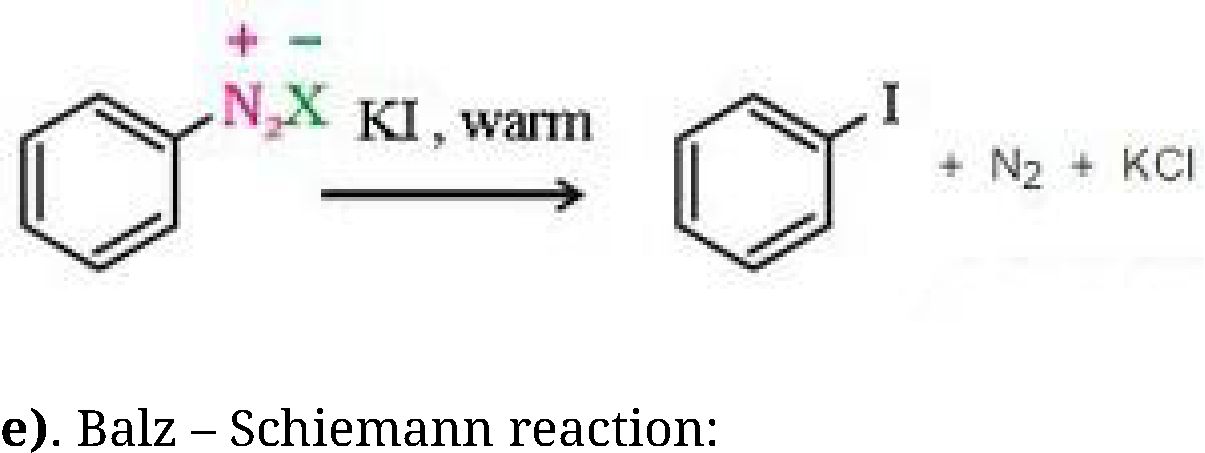
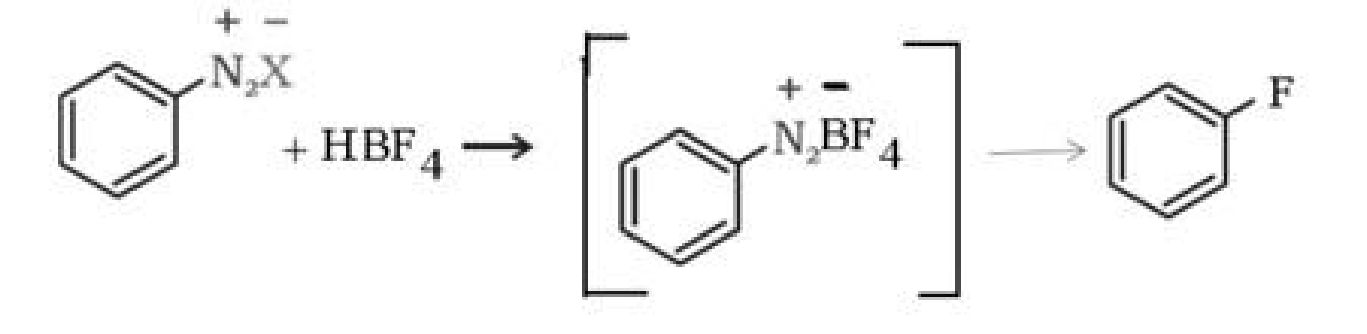
b) Sandmeyer’s reaction:
Physical properties ofhaloalkanes:
a) Solubility
Although haloalkanes are polar in nature, yet they are practically very slightly soluble in
water.
In order for a haloalkane to dissolve in water, energy is required to overcome the
attractions between the haloalkane molecules and break the hydrogen bonds between
water molecules.
However Haloalkanes are not able to form hydrogen bonds with water and therefore,
less energy is released when new attractions are set up between the haloalkane and the
water molecules because these are not as strong as the original hydrogen bonds in water
molecules.
• Chemical properties of haloalkanes:
Nucleophilic substitution reaction:
_ \8+ S“
\ ^
.C-Nu + X
*?
Reaction:
/
Mechanism of Nucleophilic Substitution
SN1 Mechanism
SN2 Mechanism
R – X + NH3 ^> R – NH2 + HX
dry ether
a) Dow’s Process

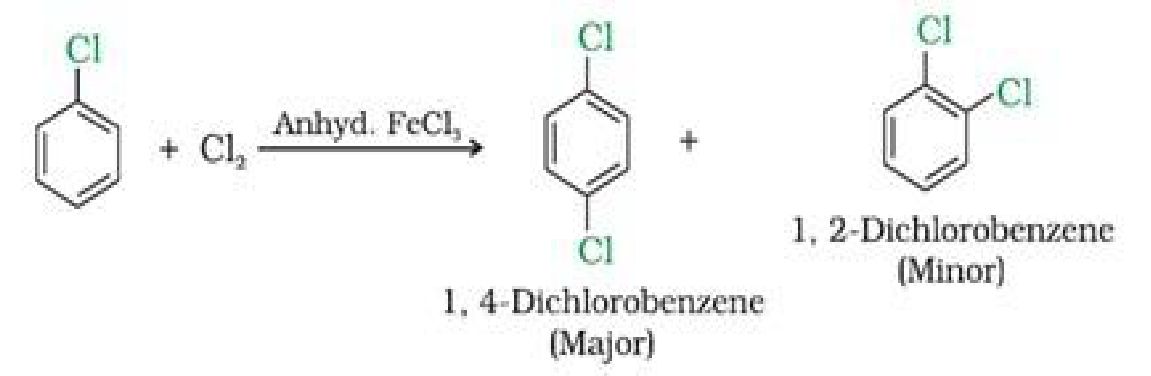
b) With halogens
c) With conc. nitric and sulphuric acid
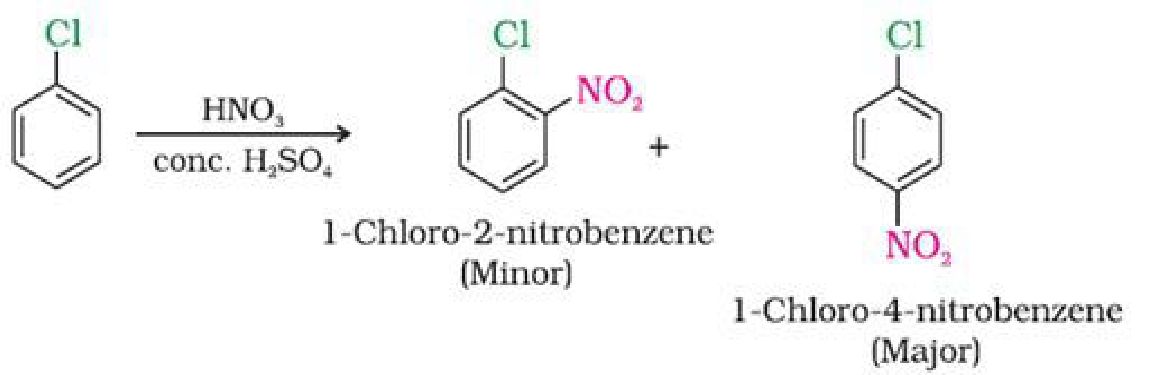
d) On heating with conc. sulphuric acid

e) With methyl chloride
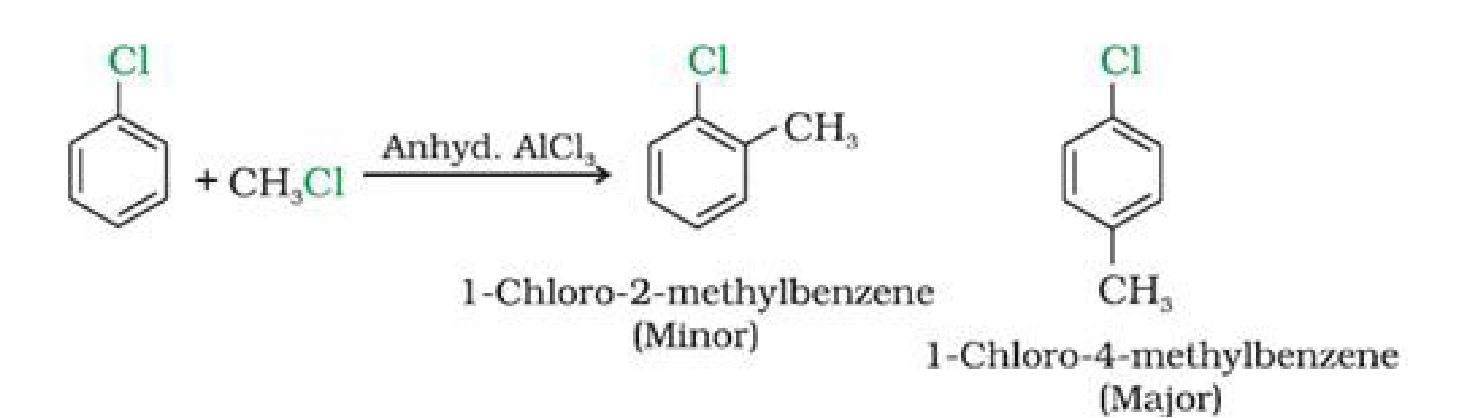
f) With acetyl chloride