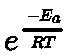CBSE Class 12 Chemistry
Quick Revision Notes
Chapter 7
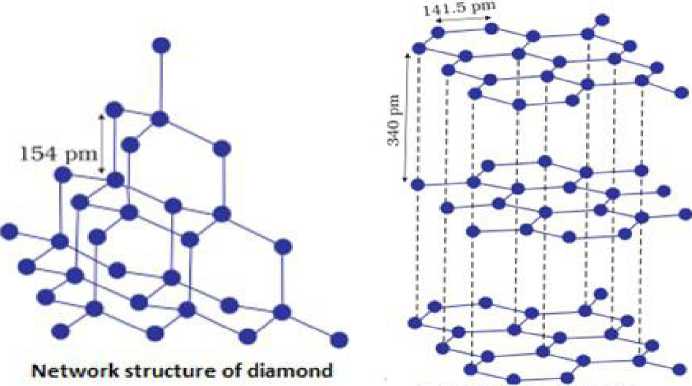
- The p-Block elements: Elements belonging to groups 13 to 18 of the periodic table are called p-block elements.
- General electronic configuration of p-block elements: The p-block elements are characterized by the ns2np1-6 valence shell electronic configuration.
- Representative elements: Elements belonging to the s and p-blocks in the periodic table are called the representative elements or main group elements.
- Inert pair effect: The tendency of ns2 electron pair to participate in bond formation decreases with the increase in atomic size. Within a group the higher oxidation state becomes less stable with respect to the lower oxidation state as the atomic number increases. This trend is called ‘inert pair effect’. In other words, the energy required to unpair the electrons is more than energy released in the formation of two additional bonds.
- Nitrogen family: The elements of group 15 – nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb) and bismuth (Bi) belong to configuration is ns^njP.
- Atomic and ionic radii:
- Covalent and ionic radii increase down the group.
- There is appreciable increase in covalent radii from N to P.
- There is small increase from As to Bi due to presence of completely filled d or f orbitals in heavy elements.
- It goes on decreasing down the group due to increase in atomic size.
- Group 15 elements have higher ionisation energy than group 14 elements due to smaller size of group 15 elements.
- Group 15 elements have higher ionization energy than group 16 elements because they have stable electronic configuration i.e., half-filled p-orbitals.
- Nitrogen shows catenation to some extent due to triple bond but phosphorus shows catenation to maximum extent.
- The tendency to show catenation decreases down the group.
- The common oxidation states are +3, +5 and -3.
- The tendency to show -3 oxidation state decreases down the group because of decrease in electronegativity by the increase in atomic size.
- The stability of +5 oxidation state decreases whereas stability of +3 oxidation state increases due to inert pair effect.
- Nitrogen shows oxidation states from -3 to +5.
- Nitrogen and phosphorus with oxidation states from +1 to +4 undergo oxidation as well as reduction in acidic medium. This process is called disproportionation.
- All group 15 elements from trihydrides, MH3.
- It belongs to sj? hybridisation.
- The stability of hydrides decreases down the group due to decrease in bond dissociation energy down the group.
NH3 > PH3 > AsH3 > SbH3 > BiH3
PH3 < AsH3 < NH3 < SbH3 < BiH3
- Boiling point increases with increase in size due to increase in van der Waals forces.
- Boiling point of NH3 is more because of hydrogen bonding.
NH3 (107.8°) > PHs (99.5°) > AsH3(91.8°) w SbHs (91.3°) > BiHs (90°)
- Electronegativity of N is highest. Therefore, the lone pairs will be towards nitrogen and hence more repulsion between bond pairs. Therefore bond angle is the highest. After nitrogen, the electronegativity decreases down the group.
- Basicity decreases as NH3> PH3> AsH3> SbH3< BiH3. This is because the lone pair of electrons are concentrated more on nitrogen and hence the basicity will be maximum in the case of NH3. It will decrease down the group as the electronegativity decreases down the group. The reducing power of hydrides increases down the group due to decrease in bond dissociation energy down the group.
- All group 15 elements from trioxides (M2Os) and pentoxides (M2O5).
- Acidic character of oxides decreases and basicity increases down the group. This is because the size of nitrogen is very small.
- It has a strong positive field in a very small area. Therefore, it attracts the electrons of water O-H bond to itself and release H+ ions easily.
- As we move down the group, the atomic size increases and so, the acidic character of oxide decreases and basicity increases down the group.
Group 15 elements form trihalides and pentahalides.
- Trihalides: These are covalent compounds and become ionic down the group with sp3 hybridisation, pyramidal shape.
- Pentahalides
- . They are lewis acids because of the presence of vacant d – orbitals.
- . They possess sjPd hybridisation and hence possess trigonalbirpyamidal shape.
- PCl5 is ionic in solid state and exist as [PCl/{\+[PClQ\~
- In PCl5, there are three equatorial bonds and two axial bonds. The axial bonds are longer than equatorial bonds because of greater repulsion from equatorial bonds.
- Nitrogen does not form pentahalides due to absence of d- orbitals.
- Reactivity towards metals: All elements react with metals to form binary compounds in -3 oxidation state.
- Anomalous behaviour of nitrogen: The behaviour of nitrogen differs from rest of the elements.
- It has a small size.
- It does not have d – orbitals
- It has high electronegativity
- It has high ionization enthalpy
Heat
NHiCl{aq) + NaNO2 (aq) ► N2(g) + 2 H2O(I) + NaCl{aq)
Heat
(NH^)2Cr2Oi y N2 + IH2O + Cr2Os
Heat
- Physical Properties:
- It is a colourless, odourless, tasteless and non – toxic gas.
- It is chemically un-reactive at ordinary temperature due to triple bond in N = N which has high bond dissociation energy.
- Ammonia molecule is trigonal pyramidal with nitrogen atom at the apex.
- It has 3 bond pairs and 1 lone pair.
- N is sp^ hybridised.
- Preparation:
Haber’s process:
Pressure 200×10 Pa Temperature 773 K Catalyst is FeO with small amounts of K2O and
Ostwald Process: The NO thus formed is recycled and the aqueous HNOs can be concentrated by distillation upto ~ 68% by mass. Further concentration to 98% can be achieved by dehydration with concentrated H2SO^ . Nitric acid is strong oxidizing agent in the concentrated as well as in the dilute state.
Pt/Rh gauge 500k, 9 bar
ANHs + 502 ► ANO + QH2O
3NO2(9) + H2O(I) ^ 2HNOs(aq) + NO(g)
- It shows the property of catenation to maximum extent due to most stable P – P bond.
- It has many allotropes, the important ones are:
- White phosphorus
- Red phosphorus
- Black phosphorus
- Discrete tetrahedral P4 molecules
- Very reactive
- Glows in dark
- Translucent waxy solid
- Soluble in C/S2but insoluble in water
- It has low ignition temperature, therefore, kept under water
- Polymeric structure consisting of chains of P4 units linked together
- Less reactive than white phosphorus
- Does not glow in dark
- Has an iron grey lustre
- Insoluble in water as well as CS2
- Exists in two forms – a black phosphorus and /3 black phosphorus
- Very less reactive
- Has an opaque monoclinic or rhombohedral crystals
573k in an inert atmosphere for several days
White phosphorus > Red phosphorus
High pressure,473K
White phosphorus > Black phosphorus
In a sealed tube,803K
Red phosphorus > Black phosphorus
- It is highly poisonous, colourless gas and has a smell of rotten fish.
- Preparation
Ca3P2 + QH2 O ^ 3Ca(OH)2 + 2PH3
Calcium Water Calcium Phosphine
Phosphide Hydroxide
Ca3P2 + QHCl ^ ZCaCl2 + 2PH3
Phosphine
P4 + 3NaOH + SH2 O ^ 3NaH2 PO2 + PH3
Sodium Phosphine
Hypophosphite
- It is a colourless oily liquid.
- Preparation
P4 + IOCl2 ^ APCh
P4 + 10SO2Cl2 ^ APCl3 + 10502
- With water,
It gets hydrolysed in the presence of moisture.
- Pyramidal shape, sp3 hybridisation
- With acetic acid
3CH3 COOH + PCl3 ^ CH3 COCl + H3PO3
- . With alcohol
3C2HhOH + PCl3 ^ ZC2H3Cl + H3PO3
C2H3OH + PCl3 ^ C2H3Cl + POCl3 + HCl
- Yellowish white powder.
- Trigonalbipyramidal shape, sp3dhybridisation .
- Preparation
4.
- With water
- PCl5 + H2O -> POCl3 + 2HCl POCl3 + 3H2O ^ H3PO4 + 3HCl
- With acetic acid
- ZCH3 COOH + PCl3 -± CH3 COCl + POCl3 + HCl
- With alcohol 10. With metals
2Ag + PCl5 ^ 2AgCl + PCl3 Sn + 2PCk ^ SnCl4 + 2PCl3
GROUP 16 ELEMENTS • Oxidation states:
- They show -2, +2, +4, +6 oxidation states.
- Oxygen does not show +6 oxidation state due to absence of d – orbitals.
- Po does not show +6 oxidation state due to inert pair effect.
- The stability of -2 oxidation state decreases down the group due to increase in atomic size and decrease in electronegativity.
- Oxygen shows -2 oxidation state in general except in OF2and O2F2
- Thus, the stability of +6 oxidation state decreases and +4 oxidation state increases due to inert pair effect.
- Ionisation enthalpy of elements of group 16 is lower than group 15 due to half-filled p- orbitals in group 15 which is more stable.
- However, ionization enthalpy decreases down the group.
- Oxygen has less negative electron gain enthalpy than S because of small size of O.
- From S to Po electron gain enthalpy becomes less negative to Po because of increase in atomic size.
- It increases with increase in atomic number.
- Oxygen has much lower melting and boiling points than sulphur because oxygen is diatomic (O2 ) and sulphur is octatomic (S%).
- All group 16 elements form hydrides.
- They possess bent shape.
- Bond angle:
- Acidic nature: H2O < H2S < H2Se < H2Te
This is because the H-E bond length increases down the group. Therefore, the bond dissociation enthalpy decreases down the group.
- Thermal stability: H2 O < H2 S < H2 Se < H2 Te < H2 Po
This is because the H-E bond length increases down the group. Therefore, the bond dissociation enthalpy decreases down the group.
- Reducing character: H2 O < H2 S < H2 Se < H2 Te < H2 Po
This is because the H-E bond length increases down the group. Therefore, the bond dissociation enthalpy decreases down the group.
- Reactivity with oxygen: EO2 and EOs
- Reducing character of dioxides decreases down the group because oxygen has a strong positive field which attracts the hydroxyl group and removal of H+ becomes easy.
- Acidity also decreases down the group.
- SO2 is a gas whereas SeO2 is solid. This is because SeO2 has a chain polymeric structure whereas SO2 forms discrete units.
- Reactivity with halogens: EX2, EX4 and EX6
- The stability of halides decreases in the order F— > Cl- > Br- > I—.
- This is because E-X bond length increases with increase in size.
- Among hexa halides, fluorides are the most stable because of steric reasons.
- Dihalides are sp3 hybridised and so, are tetrahedral in shape.
- Hexafluorides are only stable halides which are gaseous and have sj?d? hybridisation and octahedral structure.
- H2O is a liquid while H2S is a gas. This is because strong hydrogen bonding is present in water. This is due to small size and high electronegativity of O.
The compounds of oxygen and other elements are called oxides.
Heat/MnO2
Finely divided metals
2H2O2(a,q) >■ 2 H2O(l) + O2(g)
Heat
A
Re d lead
SO2 + H2O ^ H2SO3(Sulphurous acid)
Na2O + H2O ^ 2NaOH K20 + H20^2K0H CaO + H2O —> Ca(OH)2{/tex}
- Acidic oxides: Non- metallic oxides are usually acidic in nature.
- Basic oxides: Metallic oxides are mostly basic in nature. Basic oxides dissolve in water forming bases e.g.,
- Amphoteric oxides: They show characteristics of both acidic as well as basic oxides.
Al2O3 + 6HCl(aq) ^ 2AlCh(aq) + 3H2O
Al2O3 + WaOH(aq) + 3H2O(I) ^ 2Na3[Al{OH)Q\(aq)
- Neutral oxides: These oxides are neither acidic nor basic. Example: Co, NO and N2O
- Preparation: It is prepared by passing silent electric discharge through pure and dry oxygen 10 – 15 % oxygen is converted to ozone.
302(g) ^ 203(g)]AH = +142fcJmol-1
- Structure of Ozone: Ozone has angular structure. Both O = O bonds are of equal bond length due to resonance.
1. Sulphur exhibits allotropy:
- Yellow Rhombic (a – sulphur)
- Monoclinic (/?- sulphur)
369K
- a — sulphur > |3 — sulphur
- At 369 K both forms are stable. It is called transition temperature.
- Both of them have S8 molecules.
- The ring is puckered and has a crown shape.
- Another allotrope of sulphur – cyclo Se ring adopts a chair form.
- S2is formed at high temperature (~1000 K).
- It is paramagnetic because of 2 unpaired electrons present in anti bonding7T[1] orbitals like
O2.
By contact process
|S8 + 02^S02
V2O5/2bar 720k
2502 (g) + O2 (g) ► 2 SO3 {g)
AH6 = -196.6feJ mol-1
SOs (g) + H2SO4 ^ H2S2O7(oleum)
(96-98%)
- Preparation:
- Exothermic reaction and therefore low temperature and high pressure are favourable.
- It is dibasic acid or diprotic acid.
- It is a strong dehydrating agent.
- It is a moderately strong oxidizing agent.
- Atomic and ionic radii: Halogens have the smallest atomic radii in their respective periods because of maximum effective nuclear charge.
- Halogens have maximum negative electron gain enthalpy because these elements have only one electron less than stable noble gas configuration.
- Electron gain enthalpy becomes less negative down the group because atomic size increases down the group.
- These elements are highly electronegative and electronegativity decreases down the group.
- They have high effective nuclear charge.
- Bond dissociation enthalpy follows the order: Cl2> Br2> F2> I2
- This is because as the size increases bond length increases.
- Bond dissociation enthalpy of Cl2 is more than F2 because there are large electronic repulsions of lone pairs present in F2.
- Colour: All halogens are coloured because of absorption of radiations in visible region which results in the excitation of outer electrons to higher energy levels.
- Oxidising power:
- All halogens are strong oxidisingagents because they have a strong tendency to accept electrons.
- Order of oxidizing power is: F2 > Cl2 > Br2 > I2
- Acidic strength: HF <HCl<HBr< HI
- Stability: HF >HCl>HBr> HI. This is because of decrease in bond dissociation enthalpy.
- Boiling point: HCl<HBr< HI < HF. HF has strong intermolecular H bonding. As the size increases van der Waals forces increases and hence boiling point increases.
- % Ionic character: HF >HCl>HBr> HI Dipole moment: HF >HCl>HBr> HI. Electronegativity decreases down the group.
- Reducing power: HF <HCl<HBr< HI
- Halogens react with metals to form halides.
- Ionic character: MF >MCl>MBr> MI. The halides in higher oxidation state will be more covalent than the one in the lower oxidation state.
Reactivity of halogens towards other halogens:
- Binary compounds of two different halogen atoms of general formula X Xh are called interhalogen compounds where n = 1, 3, 5, or 7. All these are covalent compounds.
- Interhalogen compounds are more reactive than halogens because X-X is a more polar bond than X-X bond.
- All are diamagnetic.
- Their melting point is little higher than halogens.
- XX’ (CIF, BrF, BrCl, ICl, IBr, IF) (Linear shape) XX’3 (CIF3,BrF3, IF3, ICl3) (Bent T- shape) XX’s -CIF5,BrF5, IF$, (square pyramidal shape) XX’7 -IFj (Pentagonal bipyramidal shape)
- Fluorine forms only one oxoacid HOF (Fluoric (I) acid or hypofluorous acid) due to high electronegativity.
- Acid strength:
- Reason:
- Acid strength: HOF >HOCl>HOBr> HOI. This is because Fluorine is most electronegative.
- They have very high ionization enthalpy because of completely filled orbitals.
- Ionisation enthalpy decreases down the group because of increase in size.
- Atomic radii: Increases down the group because the number of shells increases down the group.
- Electron gain enthalpy: They have large electron gain enthalpy because of stable electronic configuration.
- Melting and boiling point: It has low melting and boiling point due to the presence of only weak dispersion forces.
- Shapes:
XeF2 is linear, XeF4 is square planar and XeFft is distorted octahedral. KrF2 is known but no true compound of He Ne and Arare known.
673fe,16ar
- Xe + F2 y XeF2
873k/7bar
- Xe d– 2F2 y XeF4
573fc/60-706ar
- Xe + SF2 > XeFft
- XeF4 + O2F2 ^ XeFft + O2
XeF2, XeF4 and XeFft are powerful fluorinating agents.
QXeF4 + UH2O ^ 4Xe + 2XeO3 + 24HF + 3O2 XeFft + 3H2O ^ XeO3 + QHF
-
Ionisation enthalpy: They have very high ionization enthalpy because of small size as compared to other groups. ↑