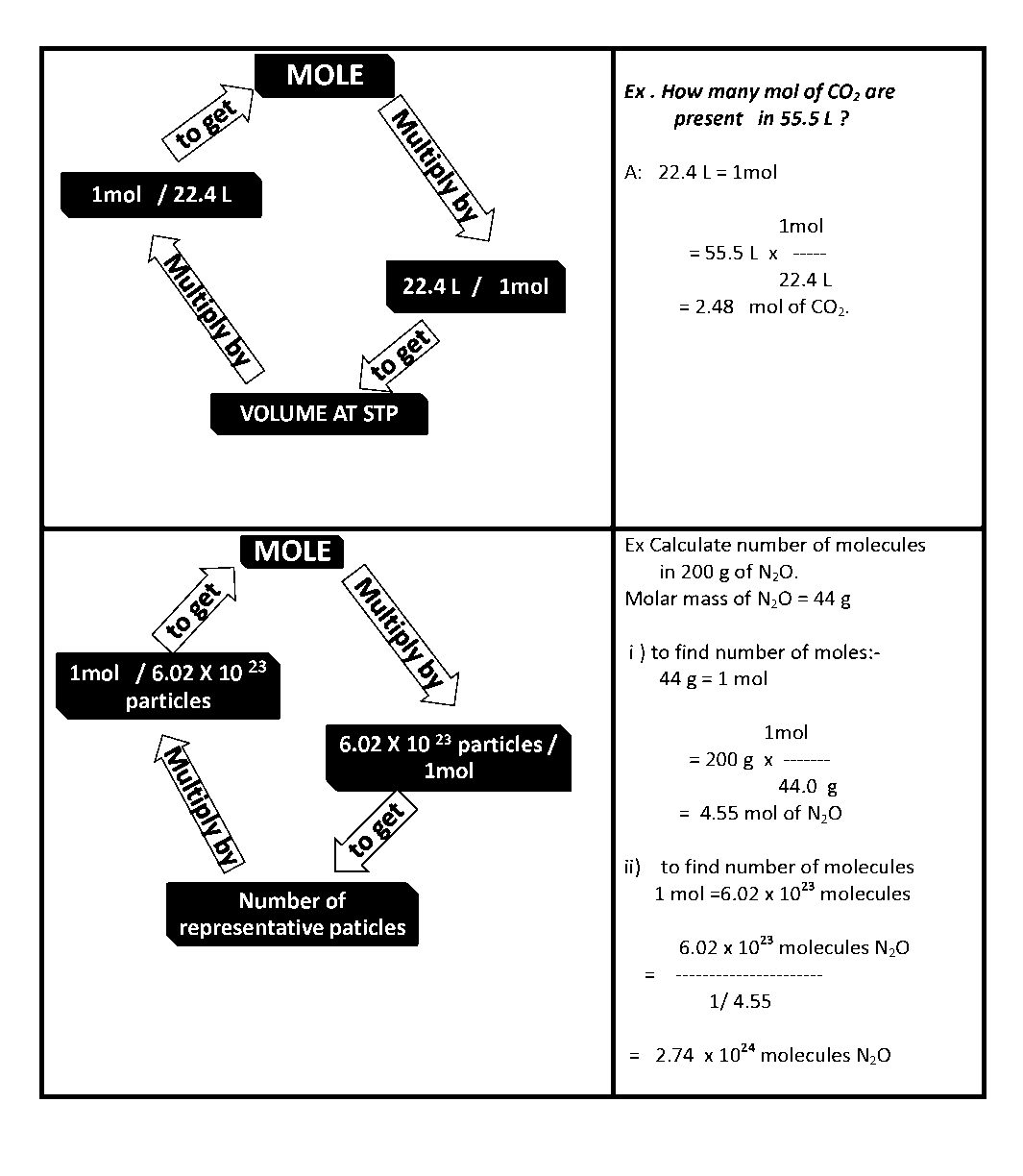
The Invisible Man
Protagonist and Antagonist
The story contains both external and internal conflict. In either case, both the protagonist and the antagonist is Griffin himself as he has made himself his own worst enemy. The external conflicts that Griffin causes are between Griffin and various members of the town as his invisibility is gradually discovered. People react with fear and then with terror as Griffin aggravates the situation by lashing out against people as soon as they figure him out. The people accept his existence with surprising lack of suspicion about the possibility of such an occurrence, which may be a lack on the author’s part. Once they believe that he exists, the primary goal is to apprehend and imprison him. Although motives are not elaborated upon, it would seem that different people in the town have different notions of what they might do when and if they could capture the man. Griffin also ultimately sees Kemp as an enemy although he had at first believed that Kemp would be both sympathetic and cooperative.
The most important conflict is internal as Griffin himself struggles to live with his situation. He rationalizes his crimes rather than making any sane attempt to get people to understand his predicament. He uses force to get people to help him and goes from bad to worse in his attempts to replenish his research materials for experiments in reversing the process that rendered him invisible. There is no real depth of character. Griffin simply runs from place to place trying to survive by increasingly decadent methods.
Climax
The climax occurs when Griffin returns to Kemp’s house intending to make an example of Kemp for having betrayed him. Kemp escapes out the window but is soon followed by Griffin who can see him although he can’t see Griffin. The entire town is soon involved in the chase.
Outcome
The resolution is the death of Griffin. Once Kemp realizes what is happening he slows down and allows Griffin to catch him. Although Kemp is buffeted about a good bit for his efforts, Griffin is weaker than usual due to his injuries. Some of the men of the town are able to grasp invisible wrists and ankles and hold him down until the effort is no longer necessary.
Synopsis
The plot is simple and straightforward. Griffin, having rendered himself invisible with an earlier experiment, enters a town and sets up a lab in an inn where he works night and day to come up with a formula that will reverse his invisibility. When he slips up and accidentally reveals himself, he engages in immature and violent actions until he is forced
Downloaded From ImperialStudy
to run and find a new hiding place. As more people become aware of his existence, his situation becomes more perilous. Finally, he stumbles into the home of a former college professor whom he assumes will be interested in his experiments and willing to help him. The doctor, Mr. Kemp, however, reads newspaper accounts of Griffin’s insane actions against people in the town and betrays his trust. Griffin is hunted down, caught and killed, whereupon he becomes visible again. The little, inconspicuous victim of some of Griffin’s behavior is left with the stolen money and the documents that explain Griffin’s experiments. The story closes with the suggestion that Marvel himself might try the experiments if only he could figure them out.
CHAPTER 1 The Strange Man’s Arrival
A stranger arrives in Bramblehurst railway station. He is bundled from head to foot with only the tip of his nose showing. He enters the Coach & Horses Inn and demands a room and a fire. Mrs. Hall, the owner prepares a supper for him and offers to take his coat and hat, but he refuses to take them off. When he finally removes the hat, his entire head is swathed in a bandage. Mrs. Hall thinks he has endured some accident. She tries to get him to talk about himself, but he is taciturn with her, although not particularly rude.
Notes – This introduction to the Invisible Man through the eyes of the town people is actually about midway through his own story. He has already gone from place to place trying to keep his cover and has committed two acts of violence, one against his own father and the other against the proprietor of a costume shop whom he tied and gagged in order to be able to steal clothing and money. Nevertheless, his intention at this point is simply to find a quiet place and work as quickly as possible to find an antidote to the invisibility. The primary thread of the story-that of the growing rumors and suspicions, which eventually contribute to his exposure-is begun.
CHAPTER 2 Mr. Teddy Henfrey’s First Impressions
Teddy Henfrey, a clock repairman, comes to the inn for tea. Mrs. Hall asks him to “repair the clock” in the stranger’s room. Teddy deliberately takes as long as he can with the clock, taking it apart and reassembling it for no reason. The stranger finally gets him to hurry up and leave. Offended, Teddy talks himself into believing that the stranger is someone of a suspicious nature, perhaps even wanted by the police and is wrapped up to conceal his identity. Teddy runs into Mr. Hall and warns him about the stranger, informing him that a “lot of luggage” will be coming. It would seem that the stranger intends to stay awhile.
Mr. Hall goes home intending to investigate the stranger, but is put off by the short- tempered demeanor of his wife.
Downloaded From ImperialStudy
Notes – Mrs. Hall, although not a major character, is revealed as rather devious in a harmless sort of way. She really wants to know what the man’s disfigurement is; she assumes he has been in a horrible accident, and the motherly side of her wants to know how to express sympathy. She is a very good innkeeper under the circumstances. While she is not above using Teddy to pry for information, she does not contribute to the spread of rumors. In fact, we are told later that she defends him as long as he is faithful about paying his bill. Teddy is a character typical of the other people of the town. He wants to know the man’s story, and when he is rebuffed for his persistence, he begins to imagine all sorts of things. His imagination soon becomes fact to him, and he spreads his new knowledge to anyone who will listen.
CHAPTER 3 The Thousand and One Bottles
A stranger arrives in Bramblehurst railway station. He is bundled from head to foot with only the tip of his nose showing. He enters the Coach & Horses Inn and demands a room and a fire. Mrs. Hall, the owner prepares a supper for him and offers to take his coat and hat, but he refuses to take them off. When he finally removes the hat, his entire head is swathed in a bandage. Mrs. Hall thinks he has endured some accident. She tries to get him to talk about himself, but he is taciturn with her, although not particularly rude.
The stranger’s luggage arrives at the inn. Numerous crates fill the deliveryman’s cart, some of them containing bottles packaged in straw. Fearenside, the cartman, owns a dog that starts to growl when the stranger comes down the steps to help with the boxes. The dog jumps for the stranger’s hand, but misses and sinks his teeth in a pant leg. The dog tears open the trouser leg, whereupon the stranger goes quickly back into the inn and to his room.
Concerned about the possibility of injury, Mr. Hall goes to the stranger’s room. He gets a glimpse of what seems like a white mottled face before he is shoved by an unseen force back through the door. The stranger soon reappears at the door, his trousers changed, and gives orders for the rest of his luggage. The stranger unpacks 6 crates of bottles, which he arranges across the windowsill and all the available table and shelf space in the inn’s parlor-a space he seems to have commandeered for himself.
Mrs. Hall enters later to tend to his needs and catches a quick glimpse of him without his glasses. His eyes seem hollow; he quickly puts his glasses on. She starts to complain about the straw on the floor, but he tells her to put it on the bill and to knock before entering his rooms. She points out that he could lock his door if he doesn’t want to be bothered, advice that he takes. He then works behind the locked door all afternoon. At one point, Mrs. Hall hears him raving about not being able to “go on.” She hears a sound like a bottle being broken. Later she takes him tea and notes the broken glass and a stain on the floor. He again tells her to “put it on the bill.”
Downloaded From ImperialStudy
Meanwhile Fearenside talks in the beer shop of Iping Hangar. Fearenside says that the stranger is a “black man,” an assumption derived from the absence of “pink flesh” when the trouser leg was ripped open. When reminded of the pink nose, Fearenside claims that the man must therefore be a “piebald,” or a part white, part black creature.
Notes – Fearenside is more observant than even he realizes. Of course, Griffin knows that a close look at his torn pant leg will reveal a “missing” leg, but he also needs to get away from the dog until they can get the animal under control. Subtle differences among characters of the town are beginning to be revealed. Mrs. Hall notices a “hollow” look to the guest’s eyes, an appearance masked by the dark glasses he usually wears. His frustration is over the failure of his experiments; she notes the mess he makes but cleans up after him with minimal complaint when he gives her extra money. Fearenside, on the other hand, liberally discusses the “discoveries” he has made as a result of the brief encounter. Fearenside refers to horses as an example of the “patchy” color that can happen when black and white are mixed.
CHAPTER 4 Mr. Cuss Interviews the Stranger
The stranger works diligently in his room until the end of April with only occasional skirmishes with Mrs. Hall. Whenever she disapproves of anything he does, he quiets her with additional payment. He rarely goes out during the day, but goes out nearly every night, muffled up regardless of the weather.
His identity becomes a topic of speculation in the town. Mrs. Hall defends him, repeating his own words that he is an “experimental investigator.” The view of the town is that he is a criminal trying to escape justice. Mr. Gould, the probationary assistant imagines that the man must be an “anarchist” who is preparing explosives.
Another group of people believe he is a piebald and could make a lot of money if he chose to show himself at the fairs. All agree, however, that due to his habits of secrecy, they dislike him. The young men begin to mock his bearing; a song called “Bogey Man” becomes popular and children follow at a distance calling out “Bogey Man.”
The curiosity of a general practitioner named Cuss is aroused, and he contrives for an interview. During the interview the stranger accidentally removes his hand from his pocket. Cuss is able to see down the empty sleeve to the elbow. Cuss questions him about “moving an empty sleeve.” The stranger laughs, then extends the empty sleeve toward Cuss’s face and pinches his nose. Cuss leaves in terror and tells his story to Bunting, the vicar.
Notes – In spite of Hall’s defense, Griffin will be the cause of his own destruction. Perhaps it is the frustration of always having to guard his secret that causes him to act offensively when challenged, but in any case, he could have handled the situation differently. The deliberate pinching of Cuss’s nose is not only an unnecessary
Downloaded From ImperialStudy
affront, but is also a mark of Griffin’s immaturity. Bringing pain upon others for the sake of his own amusement, however, will soon deteriorate to performing criminal acts. In fact, although Bunting is about to become Griffin’s new victim, Griffin has already been foraging at night for places that he could rob in order to maintain his materials and keep up with his rent.
This chapter nudges the plot forward a bit by bringing in Bunting the vicar. The actions which will follow begin to bring the town together in an awareness of a stranger in their midst.
CHAPTER 5 The Burglary and the Vicarage
Mrs. Bunting, the vicar’s wife, wakes up at the sound of bare feet walking through her house. She wakes her husband and the two watch and listen as a candle is lit and papers are rustled in the study. When they hear the telltale clink of money, Rev. Bunting rushes into the study with a raised poker, but the room appears to be empty. Their money disappears and at one point they hear a sneeze in the hallway but are unable to locate or see the intruder.
Notes – Due to the necessity of running about naked, Griffin has caught a cold, which he is unable to completely hide. His sneezes begin to give him away even though people don’t yet understand what they are hearing. In robbing the Buntings, Griffin also sets himself up for accusations and criminal charges. Thus when his presence is discovered, it is inevitable that people will begin to expect the worst and will be concentrating on apprehending him rather than helping him.
CHAPTER 6 The Furniture that Went Mad
The Halls arise very early in the morning on Whit-Monday in order to take care of some private business having something to do with their wine cellar. In passing by the guest’s room, Mr. Hall notices that the door is ajar. A few minutes later, he sees that the bolts on the front door of the house are unlocked although he remembers shutting them on the previous night. The guest is not in his room, but his clothes, shoes, and even his hat are scattered about. As the Halls are investigating, the bed-clothes suddenly gather themselves into a bundle and toss themselves over the bottom rail. Then a chair flies toward Mrs. Hall. The legs of the chair are brought to rest against her back, propelling her out of the room. The door slams and is locked behind them. The Halls decide that the stranger is a spirit.
They send for Sandy Wadgers, the blacksmith who is also supposed to be an exorcist. Wadgers is joined by Huxter, and together they ponder the likelihood of witchcraft and contemplate the propriety of breaking through the door in order to examine the situation more closely. However, before they can carry out any such action, the door opens and the stranger emerges, wrapped and bundled as usual. He distracts them long enough to enter
Downloaded From ImperialStudy
the parlor and slam the door against them. When Mr. Hall raps on the door and demands an explanation, the stranger tells him to “go to the devil” and “shut the door after you.”
Notes – The panic is building for Griffin, while characterization is enhanced for the people in the town. Wadgers delays “breaking” into the room, using the excuse of propriety when the real and very human reason is apprehension. While they may talk of spirits and witchcraft in their leisure, it is clear that they have no real experience with such. The growing impression is that the Invisible Man is something evil. Griffin helps the idea along with his continued offenses.
CHAPTER 7 The Unveiling of the Stranger
The stranger remains locked in the parlor all morning. He rings his bell for Mrs. Hall several times, but she does not answer it. About noon, he emerges and demands to know why his meals have not been brought to him. Mrs. Hall tells him that his bill has not been paid in five days. She refuses to accept the excuse that he is waiting for a remittance. When he produces some money, she refuses it, saying she first wants to know why he doesn’t enter by doorways and move about like normal people.
For his answer, the stranger removes all his head wrappings, including his nose and moustache. He thus looks like a person with a missing head. At the sound of screams a crowd of people run toward the inn. “Eye-witnesses” suddenly babble hysterical stories of the man attacking the servant girl, and brandishing a knife. Bobby Jaffers, the village constable, appears with a warrant.
The stranger slaps Jaffers with his glove, but then says he will surrender. He will not accept handcuffs, however. As the constable, Halls and others watch, the man removes the rest of his clothes, becoming invisible before them. He tells them that he is invisible. Jaffers wants to take him in for questioning on suspicion of robbing the Bunting home. A scuffle ensues, and the stranger, now known as the “Invisible Man,” escapes.
Notes – This is the last chapter in which Mrs. Hall has a significant presence, but the reader is left with the image of a very courageous, and spunky lady. She has, just a day before, been shoved out of one of her own rooms with a floating chair; she knows the man has entered and left by some mysterious means and yet she rejects his money and demands an explanation. Griffin’s own actions are quickly becoming offensive, violent and deliberately geared toward creating reactions of fear and terror in his victims. There seems to be no sense of humanity left in him; everything he does is first for survival, then for the sheer thrill of striking terror-simply because he can. He is like an evil schoolboy who enjoys pulling the legs off of flies just to see them squirm. It never occurs to him to try to solve his problem by any means other than violence and terror.
Downloaded From ImperialStudy
CHAPTER 8 In Transit
An amateur naturalist named Gibbins is relaxing out on the downs and hears someone coughing, sneezing and swearing. Frightened, Gibbins gets up and runs home.
Notes – This chapter simply indicates the passage of the Invisible Man through the countryside.
CHAPTER 9 Mr. Thomas Marvel
Marvel is an eccentric bachelor and local tramp who likes to be comfortable and take his time about things. He has come across a pair of boots in a ditch. He has tried them on and found them too big, and is occupied in contemplating the boots when he hears a voice nearby. Marvel talks about boots with the voice for several minutes before turning to see his visitor and finding no one there.
First Marvel tells himself that he has had too much to drink, then that his imagination has played some sort of trick on him. The Invisible Man begins throwing things at Marvel to convince him that he is not just imagining the presence. Eventually the Man convinces Marvel that he is real and is in need of an accomplice who will first give him food, water and shelter. He delivers an unfinished threat of what he will do if Marvel betrays him.
Notes – Marvel appears eccentric, unassuming and something of a loner, which would be bait to Griffin. He has no family, and apparently little money as he is first found contemplating whether or not he wants to keep a set of cast-off boots. He is fat, red faced, slow moving and doesn’t seem terribly bright, but that is merely the effect of Griffin having the advantage over him. As soon as he realizes his predicament, he begins to look for any possible means of escape. As for Griffin, he is “making use” of Marvel in the same way that he did the Halls, the stray cat, and even his own father. Whatever means he deems necessary to his purpose is enacted without thought or conscience.
CHAPTER 10 Mr. Marvel’s Visit to Iping
Iping has nearly recovered its earlier holiday atmosphere. As only a few people had actually made contact with the Invisible Man, the general population is soon able to reason him away as some trick of an overactive, holiday imagination.
Around 4:00, Mr. Marvel enters town and is observed by Huxter to behave rather strangely. He makes his way down the street almost reluctantly. He stops at the foot of the steps to the Coach & Horses and seems to undergo a great struggle before finally entering. A few minutes later, he re-emerges, apparently having had a drink, and walks as if he is trying
Downloaded From ImperialStudy
to act nonchalant. Soon he disappears into the yard and re-emerges with a bundle wrapped in a tablecloth. Huxter thinks some robbery has taken place and tries to follow Marvel when he is tripped in a mysterious fashion and sent sprawling.
Notes – Griffin has used Marvel to attempt to get his belongings out of the Coach & Horses. Marvel’s resistance manages to get attention, but not the attention he wants. Huxter thinks that Marvel has committed the robbery.
CHAPTER 11 In the Coach Horses
The narrator backtracks to explain what happened inside the Coach & Horses. Mr. Cuss and Mr. Bunting were in the parlor going through the belongings of the Invisible Man. Three large books labeled “Diary” are written in a cipher or code they do not understand.
Suddenly the inn door opens and Mr. Marvel enters. They disregard him and begin studying the books again when an unseen force grabs each of them by the neck and begins pounding their heads on the table between questions about what they are doing with his things. The man demands his belongings, saying he wants his books and some clothes.
Notes – Griffin is on the verge of insanity. He is probably terrified on two counts. One would be lest someone tamper with his notes or other belongings related to his experiments. The other would be lest someone should actually be able to decipher his records.
CHAPTER 12 The Invisible Man Loses His Temper
Mr. Hall and Teddy Henfrey are involved in a discussion behind the hotel bar when they hear a thump on the parlor door. They hear strange sounds as of things being thrown against the door and some bizarre conversation. Doors open and shut and they see Marvel taking off with Huxter trying to follow him. Suddenly Huxter executes a complicated leap in the air. Seconds later, Hall lands on the ground as if he had been attacked by a football player.
Several other individuals are shoved aside or sent sprawling in the streets. Mr. Cuss calls for help, telling people that the “Man” has all of the vicar’s clothes. After breaking all the windows in the Coach & Horses and thrusting a chair through the parlor window of another citizen’s house, the Invisible Man disappears from Iping.
Notes – Marvel has taken advantage of the situation, and rather than carrying Griffin’s material for him, has run off with it. The intervention of Huxter and the other individuals almost enables Marvel to get away with the precious books. Cuss quickly catches on to the fact that Griffin will be visible so long as he is carrying the bundle, but he is unaware of the existence of Marvel. The narrator tells us that “perhaps” the Invisible Man only intended to use the vicar’s clothes to cover his
Downloaded From ImperialStudy
retreat, but that at some chance blow he has “gone completely over the edge.” He throws or upends benches, chairs and boards, along with breaking windows. Eventually he catches up with Marvel and they head for the next town.
CHAPTER 13 Mr. Marvel discusses His Resignation
Mr. Marvel, propelled by the unrelenting shoulder grip and vocal threats of the Invisible Man, arrives in Bramblehurst. Marvel tries to reason his way out of the situation to no avail. The Invisible man needs a normal person to carry his books and is determined to make use of the fat, red-faced little man.
Notes – This brief chapter serves to track Griffin’s movement to the next location and to show his crude behavior toward Marvel. Marvel tries reasoning, whining, and even suggesting that he may in the long run be a failure and thus “mess up” Griffin’s plans. Nothing works. For the moment, Griffin needs Marvel. If Marvel should drop in accordance with his professed heart condition, it would mean nothing to Griffin.
CHAPTER 14 At Port Stowe
Marvel arrives in Port Stowe and is seen resting on a bench outside of town. He has the books with him, but the bundle of clothing has been abandoned in the woods. As he sits there, an elderly mariner, carrying a newspaper, sits down beside him. Citing the paper, the mariner brings up the topic of an Invisible man.
According to the newspaper, the man afflicted injuries on the constable at Iping. Certain evidence indicates that he took the road to Port Stowe. The mariner ponders the strange things such a man might be able to do-trespass, rob or even slip through a cordon of policeman.
Marvel begins to confide in the mariner, saying he knows some things about this Invisible Man. Suddenly Marvel is interrupted by an attack of some kind of pain. He says it is a toothache, then goes on to say that the Invisible Man is a hoax. Marvel begins to move off, walking sideways with violent forward jerks.
Later the mariner hears another fantastic story-that of money floating along a wall in butterfly fashion. The story is true, however. All about the neighborhood, money has been making off by the handful and depositing itself in the pockets of Mr. Marvel.
Notes – Marvel tries to take advantage of a short respite to let someone else know about the Invisible Man, but he is caught by Griffin before he can complete his story. This chapter gives us a little insight as to how Griffin has been surviving to this point. He has been stealing money wherever he could find it. Now that he is obliged to remain invisible, however, he has to use Marvel as a repository for his ill-gotten gain. The irony is that although Griffin can steal unlimited amounts, he has no way to use the
Downloaded From ImperialStudy
money in his invisible condition. And Marvel, who is for a time nothing more than a helpless victim, will be the one to benefit in the end.
CHAPTER 15 The Man Who Was Running
Dr. Kemp happens to be day-dreaming out his window when he spots a short, fat man running down the hill as fast as he can go. The doctor notices that the man is running “heavy” as if his pockets are “full of lead.” Kemp’s reaction is one of contempt, but the people on the street who see him approaching react a bit differently. The running man is Marvel; his expression is one of terror. A short distance behind him, people hear the sound of panting and a pad like hurrying bare feet. Soon cries of “The Invisible Man is coming” are heard in the streets along with the slamming of doors as people bolt into their houses.
Notes – This chapter simply introduces Kemp into the story. Kemp’s attitude is representative of the average established, self-confident, and self-sufficient individual. He sees a man in trouble, but his reaction in contemptuous instead of concern. He has heard warning cries about an Invisible Man, but clearly doesn’t believe any of it. He is a man who keeps himself apart form the concerns of the general public, is buried in his work, interested only in what award it will ultimately bring him.
CHAPTER 16 In the lolly Cricketers
The Jolly Cricketers is a tavern. The barkeep, a cabman, an American and an off-duty policeman are engaged in idle chat when marvel bursts through the door. Marvel begs for help, claiming the Invisible Man is after him.
A pounding begins at the door and then a window is broken in. The Invisible Man doesn’t come in immediately, however. The barman checks the other doors, but by the time he realizes the yard door is open, the Invisible Man is already inside. Marvel, who is hiding behind the bar, is caught and dragged into the kitchen. The policeman rushes in and grips the invisible wrist of the hand that holds onto Marvel, but is abruptly hit in the face.
People stumble over and into each other as all try to catch the Invisible Man. He yelps when the policeman steps on his foot, then flails wildly about with his Invisible fists and finally gives them the slip. The American fires five cartridges from his gun, sweeping his gun in a circular pattern as he fires. The chapter ends with the men feeling around for an invisible body.
Notes – Griffin is injured in this chapter. He is thus forced to find shelter and help in the nearest possible place. But now, enough people have been involved in Griffin’s mayhem that it will be relatively easy to round up a posse of believers when the time comes to do so.
Downloaded From ImperialStudy
CHAPTER 17 Doctor Kemp’s Visitor
Doctor Kemp is still working in his study when he hears the shots fired in the Cricketers. He opens his window and watches the crowd at the bottom of the hill for a few minutes, then returns to his writing desk. A few minutes later, he hears his doorbell ring, but his housekeeper says it was only a “runaway” ring.
The doctor is at his work until 2 AM when he decides to go downstairs for a drink. On the way, he notices a spot of drying blood on his linoleum floor. Then he finds more blood on the doorknob of his own bedroom. In his room, his bedspread is smeared with blood, his sheet is torn, and bedclothes are depressed as if someone has been sitting there.
The Invisible Man introduces himself to Kemp. He is Griffin, of University College. He explains that he made himself Invisible, but is wounded and desperately in need of shelter, clothes and food.
Kemp loans him a dressing gown along with some drawers, socks and slippers. Griffin eats everything Kemp can rustle up and finally asks for a cigar. He promises to tell Kemp the story of his bizarre situation but insists that he must sleep first as he has had no sleep in nearly three days.
Notes – Kemp’s reaction is in stark contrast to Marvel’s original reaction to Griffin. Although he finds the story hard to believe, he is too well educated and too intelligent to deny the evidence of his own eyes. Nor is he prey to hysterics or to working class superstitions. The idea of a spirit or witchcraft doesn’t even occur to him. His cool demeanor as he helps Griffin to the things he needs could be an indication of hope for the Invisible Man.
CHAPTER 18 The Invisible Man Sleeps
Griffin examines the windows of the room, then exacts a promise from Kemp that he will not be betrayed in his sleep and finally locks the door, barring Kemp from his own room.
Kemp retires to his dining room to speculate upon the strange events. There he sees the day’s newspaper, which he had ignored earlier. He reads it eagerly, but assigns the more terrifying elements of the stores to “fabrication.” In the morning, he sends his housekeeper for all available papers and reads those as well. The papers contain stories of the previous evening’s events at the Cricketers along with a rather badly written account of Marvel’s experience. Marvel doesn’t tell how he came upon the money in his pockets, nor does he mention the location of the three books. Kemp becomes alarmed at the possibilities of what Griffin could do and writes a note to Colonel Adye at Port Burdock.
Downloaded From ImperialStudy
Notes – Kemp experiences his first apprehension because of what his own intelligence reveals to him rather than from the hysterical reports in the papers. He is motivated, however, from personal interest. When he recalls the behavior of Marvel, he realizes that Marvel-a mere tramp-was being pursued by Griffin. He suddenly realizes that Griffin is insane to the point of being homicidal.
CHAPTER 19 Certain First Principles
Griffin explains how he became invisible. He had been a medical student, but had dropped medicine and taken up physics. He discovered a formula of pigments that lowers the refractive index of a substance, allowing light to pass through it rather than being reflected or refracted. After experimenting with pigments for three years, he came upon the secret whereby animal tissue could be rendered transparent. He was continuously trying to hide his work from another professor. He was finally brought to a halt in his experimenting by a lack of funds, a problem he solved by robbing his own father. Because the money did not belong to him, his father shot himself.
Notes – From this chapter through XXIII, the point of view changes as Griffin tells his own story. He explains how he became invisible and tells the story up to the time when he had first entered the Coach & Horses. He explains his use of and contempt for Marvel, justifying his own behavior as necessary to his survival.
CHAPTER 20 Doctor Kemp’s Visitor
Griffin explains how he had found lodging in a boarding house on Great Portland Street. After his father’s funeral, he went to his apartment to continue with his experiments. He successfully made a piece of cloth disappear, then he tried his process on a stray cat. The cat was not entirely successful, as the animal’s eyes and claws never completely disappeared.
Later the next day he had a minor altercation with the landlord who brought reports of Griffin tormenting a cat in the night. The landlord wanted to know what Griffin was doing in the room and what all the paraphernalia was for. The two argued and Griffin shoved the landlord out of the room. Griffin knew he would have to act quickly, so he made arrangements to have his belongings stored, then he drank some of his own potion. In the evening the landlord returned with an ejection notice, but was too terrified at the stone white face of Griffin to serve it. In spite of extreme illness and pain, Griffin finished his treatment and watched himself gradually disappear.
In the morning, the landlord, his stepsons and the elderly neighbor lady who had complained about the cat enter Griffin’s apartment and are astonished to see no one. A day later, afraid, lest his equipment reveal too much information, Griffin smashes the items and sets fire to the house. Believing that he has covered his tracks with impunity, he begins to
Downloaded From ImperialStudy
imagine all sorts of “wild and wonderful” things he will be able to do under the cover of invisibility.
Notes – Griffin’s explanations are completely absent of any sense of humanity or conscience. His intentions suggest anarchy or lawlessness resulting from an absence of social restriction. Killing his own father seems to have killed his conscience, and the novelty of invisibility highlights his immaturity and seems to divorce him from a normal sense of responsibility.
CHAPTER 21 In Oxford, Street
Griffin continues to explain his experiences with invisibility. He soon discovered that being invisible had as many drawbacks as advantages. People ran into him and stepped on him. He had to be continually on guard as to the movements and positions of others in order to avoid accidental contact. To make matters worse, although people could not see him, dogs could detect him with their keen sense of smell. As he had to remain naked, he was soon uncomfortable. Also, he could not eat, as food was visible until it was fully assimilated into his system.
At one point, he had run up the steps of a house in order to avoid a unit of a marching Salvation Army band. While he waited, two youngsters spotted the prints of his bare feet in the mud. Soon a crowd of people had gathered to look at the “ghost prints.” He leapt over the railing and ran through a bunch of back roads to avoid the press. Fortunately for him, his escape at that time was aided with the distraction created by conflagration engulfing his former dwelling.
Notes – Griffin’s initial error was that he became so obsessed with a single scientific notion that he failed to take consequences into consideration. No doubt, he was not concerned about people reacting to him as though he were some kind of mutation or monster. As an albino human, he was already a marginalized individual who did not fit into ordinary society. College was the perfect place for him, but he was so concerned about the possibility of any one getting credit for his discovery that he failed to take advantage of collaboration and more mature knowledge that he might have had access to.
CHAPTER 22 In the Emporium
Griffin explains his first attempts to get clothing and render his situation more tolerable. He had gone into the Omniums, a large apartment type store where one could buy everything from groceries to clothing. He made his way to an area of bedsteads and mattresses, hoping that once the store closed for the night, he would be able to sleep on the mattresses and steal some clothes with which to mask his condition.
Downloaded From ImperialStudy
In the night, he procured a complete set of clothes for himself, helped himself to food in a refreshment department, and then slept in a pile of down quilts. He failed to awaken before the morning crew had entered, however, and was unable to escape as long as they could see him. Thus, he was forced to shed the clothing and run, naked, back out into the cold.
Notes – Griffin was preoccupied with getting his food and clothes by illicit means. His plans are continually evil even as the reactions of other people are consistently behaviors of suspicion and rejection. At no point, does he consider trying to get anyone to understand his situation. His imagination drives him only toward evil, as if the grotesque and the evil are natural partners.
CHAPTER 23 In Drury Lane
Griffin’s peril increased daily. He had no clothes or shelter and dared not eat. Also, he soon realized that walking through the streets of London was going to result in an accumulation of dirt on his skin- which would make him visible in a grotesque way.
He made his way into a costume shop, hoping to make way with some clothes and dark glasses after the proprietor had gone to bed. In the shopkeeper’s room, he had to stand and watch the man eat his breakfast. Furthermore, the man had exceptionally acute hearing and nearly discovered Griffin several times. When evening came, he was finally able to explore the house and found a pile of old clothes. In his excitement, he forgot about the noise he was making and was nearly caught when the shopkeeper investigated the noise. Unable to see the source, but positive someone was in the house, the proprietor went about locking all the doors in the house and pocketing the keys. In desperation, Griffin struck the old man on the head, then gagged and tied him with a sheet. Then he put together a costume of old clothes, stole all the money he could find and went out into the street.
Believing his troubles were over, Griffin went into a restaurant and ordered a meal, but soon realized he couldn’t eat it without exposing his invisible face. He ordered the lunch and left, telling the proprietor that he would be back in ten minutes.
Griffin went to “another place” (which happens to be the Coach & Horses Inn) and demanded a private room, explaining that he was “badly disfigured.” Thus, he had set himself up at Iping, hoping to find a way to reverse the process of invisibility. Here he was finally discovered.
Notes – This chapter brings us current with events in the first chapter of the book.
CHAPTER 24 In Oxford, Street
Griffin tells how his original plan, after being discovered by the people of Iping, had been to get his books and get out of the country, but that plan had changed upon meeting
Downloaded From ImperialStudy
Kemp. He thinks that Kemp can work with him. Together they can set up a “reign of terror” to take full advantage of the Invisibility. Griffin does not realize that Kemp has already betrayed him and is only trying to keep him talking until the police arrive. Kemp stands in front of the window to keep Griffin from seeing the police, but Griffin soon hears them on the stairs and realizes he has been deceived.
Griffin quickly begins to disrobe even as Kemp springs to the door and attempts to lock him in. A dropped key spoils the effort as the now invisible Griffin shoves him aside, then hurls his weight at Colonel Adye, the chief of the Burdock Police who is approaching on the stairs. Griffin escapes past two more policemen in the hall; they hear the front door of the house slam violently.
Notes – In assuming that he can make demands and others will simply capitulate to him, Griffin has misjudged Kemp. Kemp is self-centered, but is not a murderer. As for Griffin himself, he appears to have abandoned any intention of searching for an antidote and is only interested in trying to terrorize as much of the country as he can. He wants to set himself up as a vindictive god with Kemp as his personal henchman.
CHAPTER 25 The Hunting of the Invisible man
Kemp explains the situation to the police, informing them of Griffin’s intentions to cause general mayhem. They talk of using dogs to sniff him out and of putting powdered glass in the streets.
Notes – The narrator tells us that if he had used his time more wisely, Griffin may have been able to escape during the 24 hours it took the countryside to organized. He slept instead, however, and by the time he had awakened there was no escape possible.
CHAPTER 26 The Wicksteed Murder
By 2:00 in the afternoon, the entire countryside around Burdock has been mobilized. Men set out with guns, clubs and dogs, and the police warn the village people to lock their doors and stay inside. Griffin manages to evade his pursuers for a 24-hour period except for one encounter with a middle-aged man who had apparently cornered him. Griffin kills the man by beating him with an iron rod.
CHAPTER 27 The Siege of Kemp’s House
Kemp receives a letter telling him that the Reign of Terror is beginning and that Kemp himself will be the first execution for the sake of an example. Kemp decides that he himself will be the bait and that Griffin will be caught because he will have gone too far. A
Downloaded From ImperialStudy
knock at the door turns out to be Adye with news that Kemp’s housekeeper-who was carrying notes for the police-had been attacked and the notes taken from her.
Griffin makes his presence known by smashing windows in Kemp’s house. During the battle that follows, Adye is shot. Griffin gets inside the house and tries to tell the police to “stand away” as he is after only Kemp. He swings an ax at them, but one of them manages to strike him with an iron poker. By this time Kemp has followed his housekeeper through a window and is nowhere to be found.
Notes – The police express contempt for Kemp, believing he has run off and left them to face Griffin alone. The truth is, he has, because he knows Griffin will follow through on his threats. However, even though Kemp tries to escape, he does not forget his earlier idea of using himself as bait. It is ironic that he runs the same course he watched Marvel run just a couple days earlier. He, too, is white faced and terrified, but keeps his wits; whenever he finds a bit of uneven ground or a patch that is scattered with broken glass, he takes it, knowing it will slow down the invisible, barefooted Griffin.
CHAPTER 28 The Hunter Hunted
Griffin chases Kemp through the town. People begin to join in the chase. When Kemp realizes that the people are chasing Griffin, he stops running, which allows the Invisible Man to catch him. Even though people cannot see him, they are able to grab hold of him and keep him down. The effort is not needed for long as Griffin has been fatally injured and seems to have lost a lot of blood. As the town people watch, the effect of invisibility is gradually reversed, and soon, Griffin, now dead, is visible.
Notes – When Griffin becomes visible, his albino condition is also revealed. It is interesting that the people are not horrified or even surprised. Nor is there any speculation about how this bizarre incident could have happened. The people watch as his broken, battered body slowly becomes visible from his extremities to the center of his being. It is only when his white face and hair and staring garnet eyes are revealed that someone calls for them to “cover that face” before the children in the town can see it.
EPILOGUE
Mr. Marvel, formerly the tramp, has become the landlord of the little inn near Port Stowe and the “owner” of all the information about Griffin. He has been able to keep all the money Griffin stole because lawyers could not identify the sources accurately. The books seem to have disappeared entirely; at least whenever anyone asks Marvel about them, he denies knowing anything. However, when the inn is closed and he is alone, he takes the books out of their hiding place and tries to study the “wonderful secrets.”
Downloaded From ImperialStudy
Notes – The epilogue implies that the people, represented by the tramp-turned-innkeeper, not only have learned very little from the experience of the invisible man, but that they would not be above trying the invisibility themselves if only they knew how to do it. Regardless of the horrors perpetrated by Griffin, it seems to be part of human nature to want to be able to cause chaos and commit obscenities with impunity. While Marvel says that he would not do the same things Griffin did, there is little doubt that anyone, given such advantage over others, would resist the temptation to dabble in behaviors that are unacceptable in normal civilized society.
Downloaded From ImperialStudy



 Effects of Electric Field on Cathode Rays
Effects of Electric Field on Cathode Rays






 Main Postulates of the Bohr Model [refer NCERT Text Book article 4.3 page number-49]
Main Postulates of the Bohr Model [refer NCERT Text Book article 4.3 page number-49]