स्मृति पाठ का सार


Hindi Sanchayan Summary Notes for Class 9th of all Chapters
Free Certification Title Name: Entrepreneurship 101 – From Idea to Launch (And Beyond)
Discover everything you need to know to become an entrepreneur and get your business up and running in no time.

Created by Master It
How to Subscribe For Entrepreneurship 101 – From Idea to Launch (And Beyond)?
Apply Coupon Code: MAYFLOWERS
**Note: Free coupon may expire soon.**
Free Certification Title Name: Complete SQL Bootcamp with MySQL, PHP & Python
Master yourself in SQL, do practical projects with MySQL, PHP and Python
Learning both SQL is one of the fastest ways to improve your career. Hope this course will be used as a helping hand for your prospective career. Please dig on free preview videos for more information.
Created by Creative Online School
How to Subscribe For Complete SQL Bootcamp with MySQL, PHP & Python?
Apply Coupon Code: BOOTCAMP
**Note: Free coupon may expire soon.**
Free Certification: English Grammar tenses & structures
Be an expert in the English grammar tenses and structures ( Active & Passive ).
Created by Ustazy
How to Subscribe For English Grammar tenses & structures?
Apply Coupon Code: MAYSECOND
**Note: Free coupon may expire soon.*
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 16
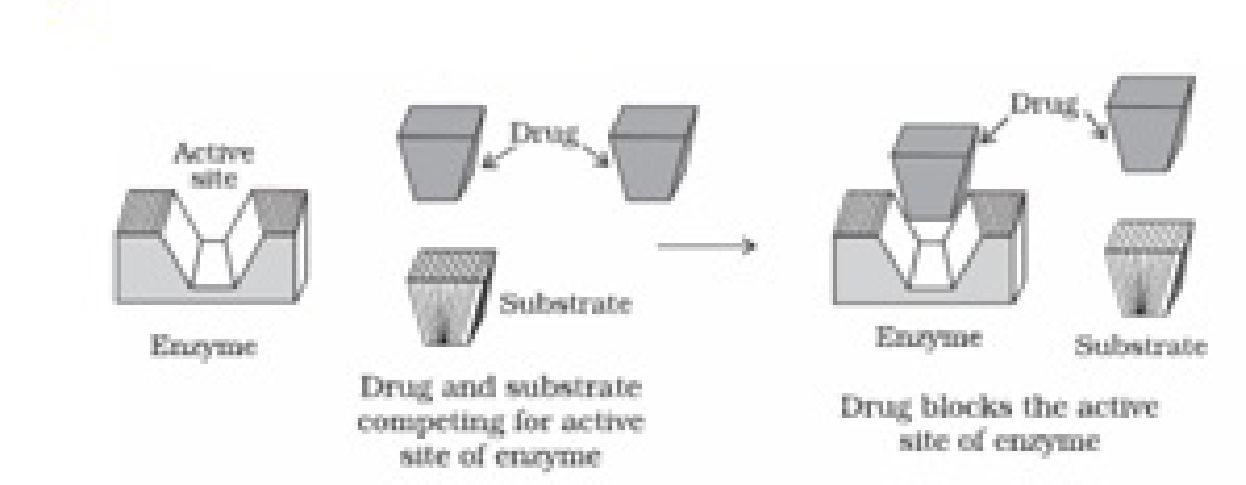
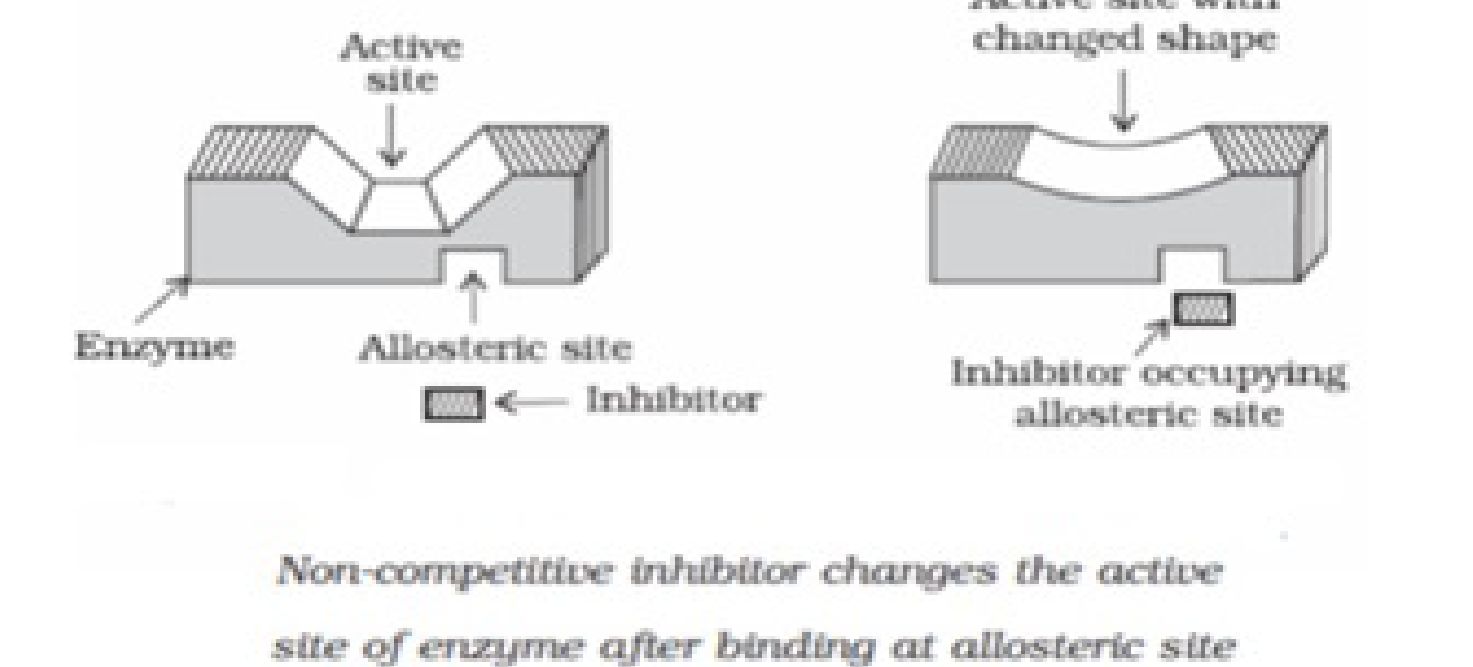
natural substrate for their attachment on the active sites of enzymes.
Ar I 1 ■ ■ r■ blli> ur| I fl
Classification of antimicrobial drugs based on the mode of control of microbial
diseases:
Classification of antimicrobial drugs based on its spectrum of action:
CH2^H
!
o
CH2– O-C-C17H3s
I O
M
CH – O – C – C17Hjs + 3NaOH
I O
J A [1 * .,
CH2– O-C-C17Hjs
Glyceryl ester Sodium
of stearic acid (Fat) hydroxide
» 3CiyHJSCOONa + CH -OH
I
(Soap) CH1^H
Sodium Gtycerol
stearate
This reaction is known as saponification.
scraping off the soaps in small broken pieces.
2C17HyCOONa-CaCl- ^ % NaCl– Ca
^oap ftGoltfbte Cd L. Lur.
::=:^i:r (ioap)
2C-H3jCOOXa+MgCl3 ^2 NaCl-(C-H3jCOO)3Mg
iQap ir.E■:■ It’Dh ™ zr.dE i.um
E-[ddfd te (EC-Erp)
These precipitates stick to the fibres of the cloth as gummy mass and block the ability of
soaps to remove oil and grease from fabrics. Therefore, it interferes with the cleansing
ability of the soap and makes the cleansing process difficult.
In acidic medium, the acid present in solution precipitate the insoluble free fatty acids which
adhere to the fabrics and hence block the ability of soaps to remove oil and grease from the
fabrics. Hence soaps cannot be used in acidic medium
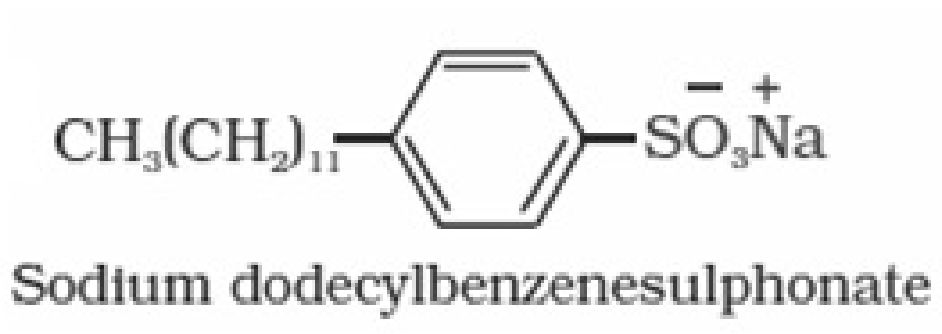
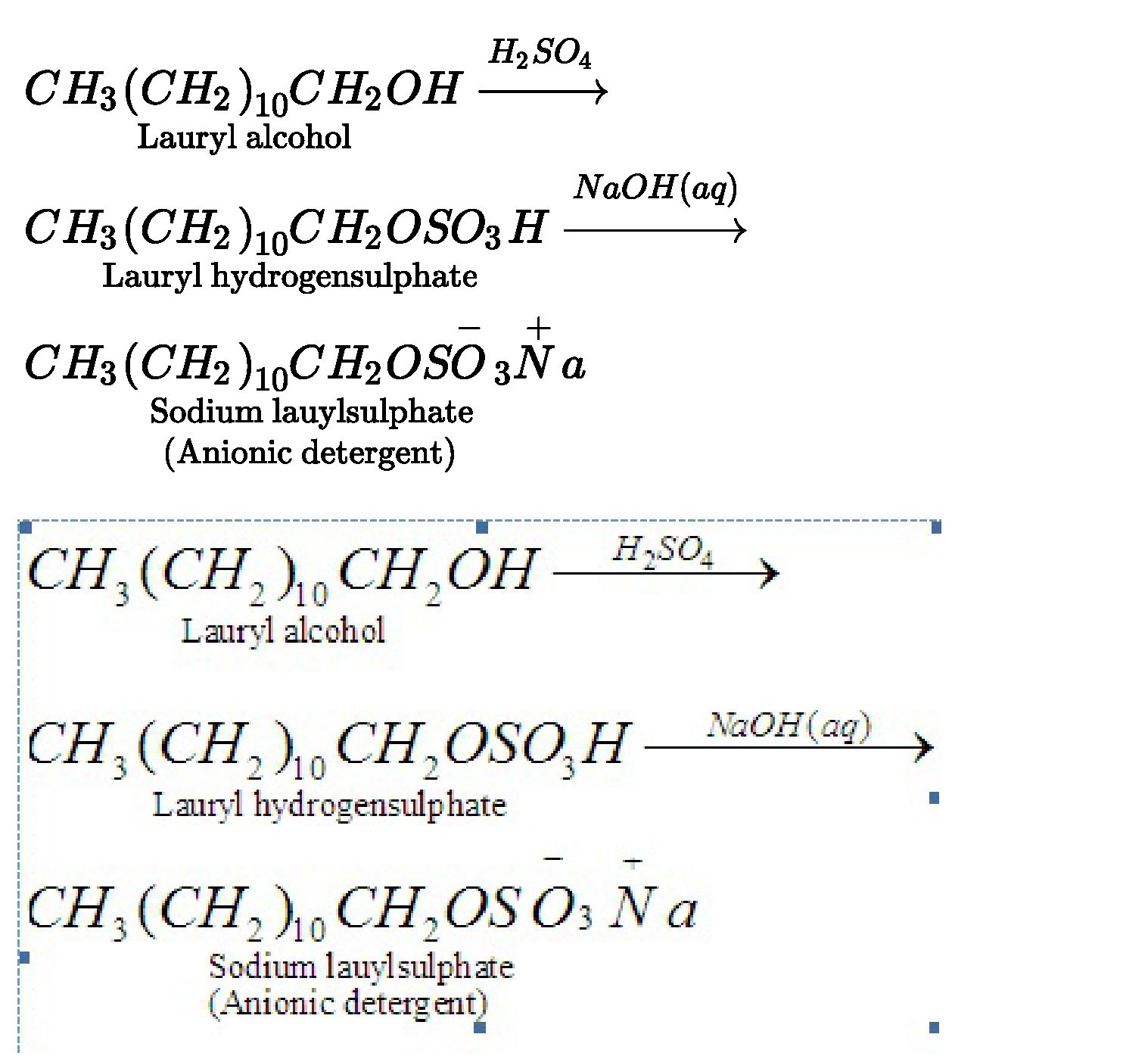
• Detergents: Detergents are sodium salts of long chain of alkyl benzene sulphonic
acids or sodium salts of long chain of alkyl hydrogen sulphates.
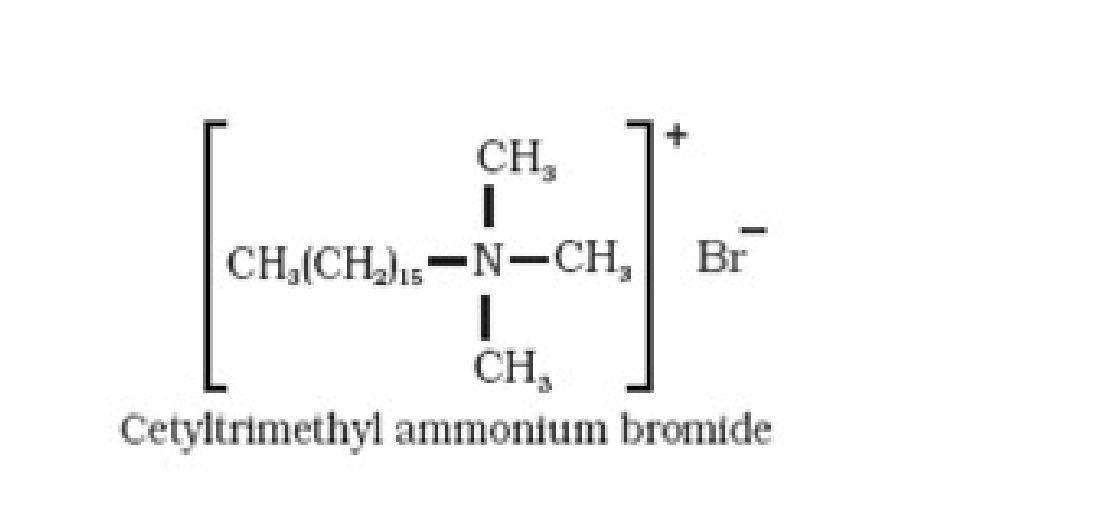
• Classification of detergents:
They are used in household cleaning like dishwasher liquids, laundry liquid detergents,
laundry powdered detergents etc. They are effective in slightly acidic solutions where soaps
do not work efficiently.
Example: Detergent formed by condensation reaction between stearic acid reacts and poly
ethyl eneglycol.
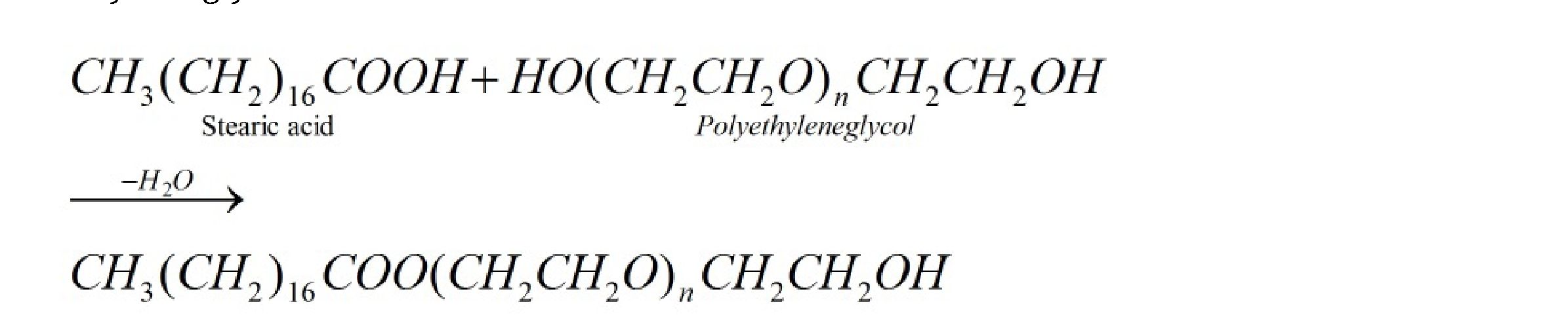
It is used in Making liquid washing detergents. They have effective H- bonding groups at one
end of the alkyl chain which make them freely water soluble.

CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 15
Polymers
Homopolymers:Polymers formed by the polymerisation of a single monomeric species.
Examples – Polythene, Polystyrene.
Copolymers:Polymers formed by addition polymerisation of two different monomers.
Examples – Buna-S, Buna -N.
Based on Molecular forces, it is classified into
Step 1: Chain initiating step: Organic peroxides undergo homolytic fission to form free
radicals which acts as initiator. Initiator adds to C-C double bond of an alkene molecule to
form a new free radical
O O O
Ii fus I il . *
<VJ,^KW>C-Crt >2C^>0^-2CA +2C0,
Bcnznyi pmaKk ETwmi redjcaJ
* ■
C*H*+CHj=CKt * CJi,-CJi:-CHL
Step 2: Chain propagating step: Free radicals formed by homolytic cleavage adds to a double
bond of monomer to form a larger free radical. Radical formed adds to another alkene
molecule to form a larger free radical. This process continues until the radical is destroyed.
These steps are called propagation steps.
CiHi ~ CH1 – C H1 – CH1 = CH1
CJii – CH1 – CH1 – CH1 – C H1
¥
CiHi – CCH1 – CH^ – CH1 – C H1
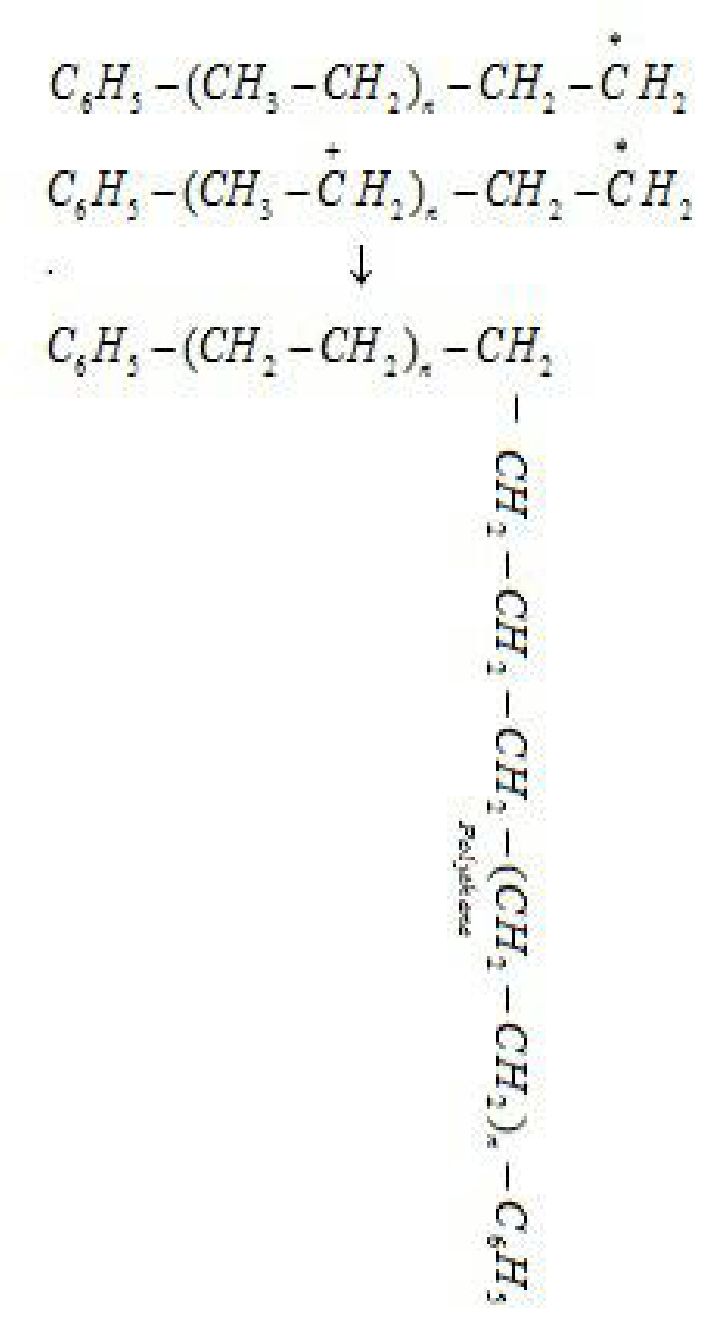
Step 3: Chain terminating step: For termination of the long chain, free radicals combine in
different ways to form polythene. One mode of termination of chain is shown as under:
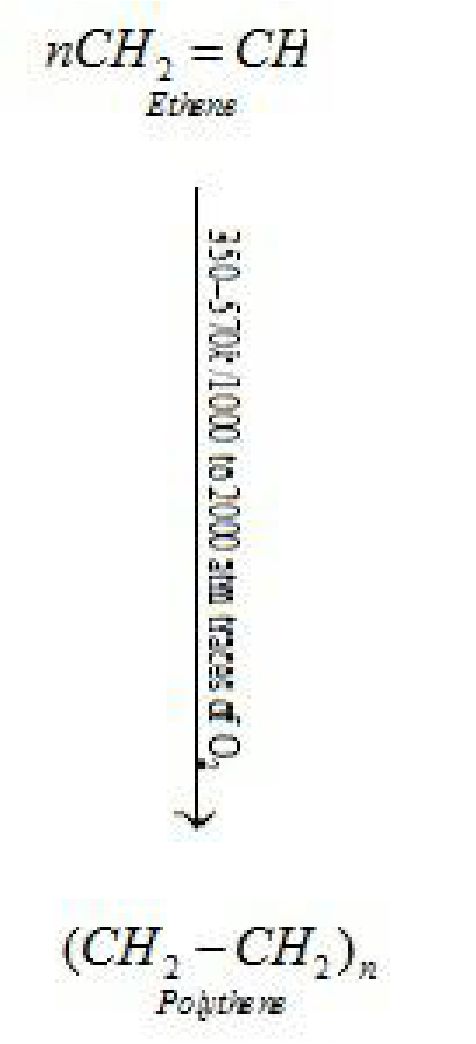
It is used in the insulation of electricity carrying wires and manufacture of squeeze bottles,
toys and flexible pipes

It is used for manufacturing buckets, dustbins, bottles, pipes, etc.
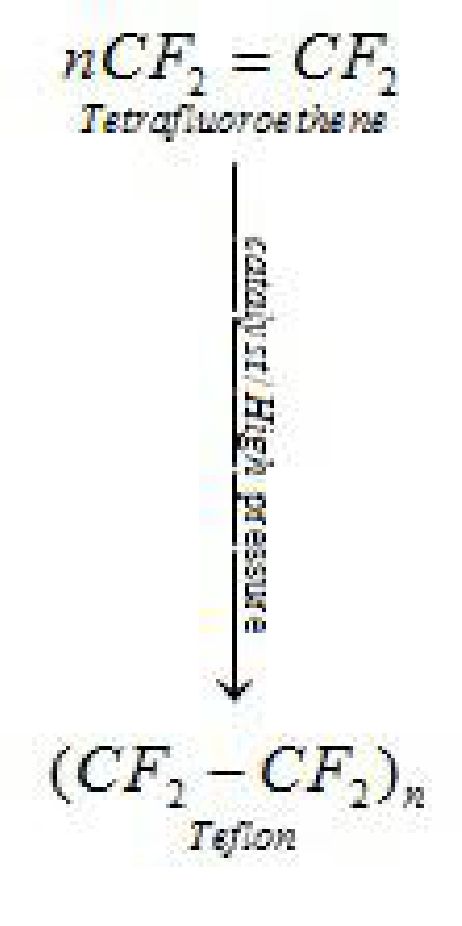
It is used in making oil seals and gaskets and also used for non – stick surface coated utensils
It is used as a substitute for wool in making commercial fibres such as orlon or acrilan.
1. Polyamides: Polymers possess amide linkage (-CONH-) in chain. Thesepolymers are
popularly known as nylons. Examples:
(a) Nylon 6, 6: It is prepared by the condensation polymerisation of hexamethylenediamine
with adipic acid under high pressure and at high temperature.
KHOOC(CH2)4COOH+ }TH2H(CH:)^XH2
It is used in making sheets, bristles for brushes and in textile industry.


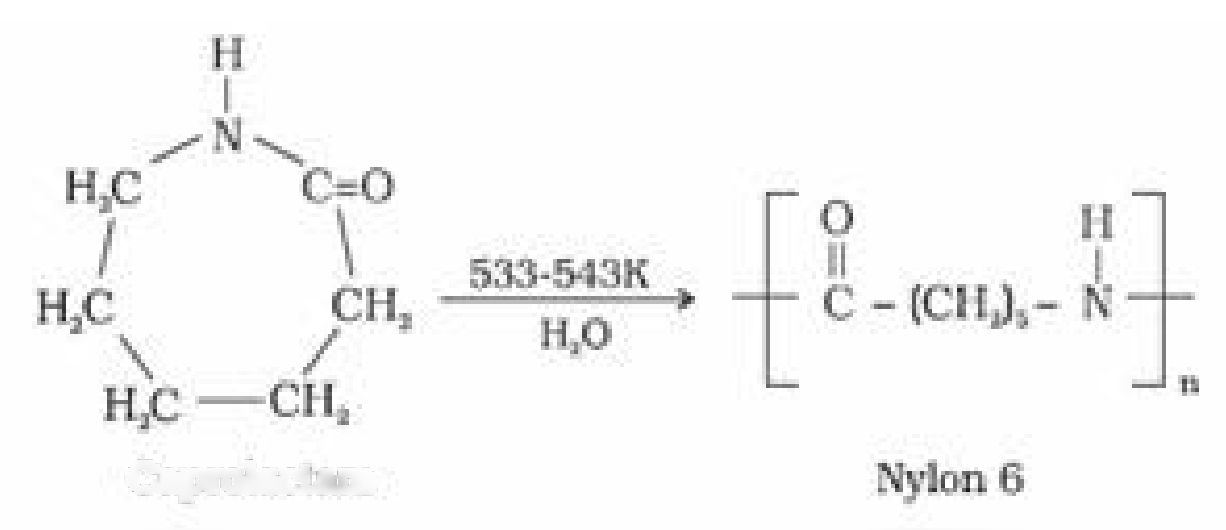
(b) Nylon 6: It is obtained by heating caprolactum with water at a high temperature
CupraUcLam
It is used for the manufacture of tyre cords, fabrics and ropes.
n HOHt- CH1OH t n HOOCn0- COOH —*
Efl^DK^ol TerepbLhabc ^rid
|Etha^!.2’dKfl (BmrKie-!.4 – dl
It is used to create resistance in polymerised product and is used in blending with cotton and
wool fibres and also as glass reinforcing materials in safety helmets, etc.
a). Bakelite: These are obtained by the condensation reaction of phenol with formaldehyde
in the presence of either an acid or a base catalyst. The initial product could be a linear
product – Novolac used in paints.

b). Novolac on heating with formaldehyde forms Bakelite
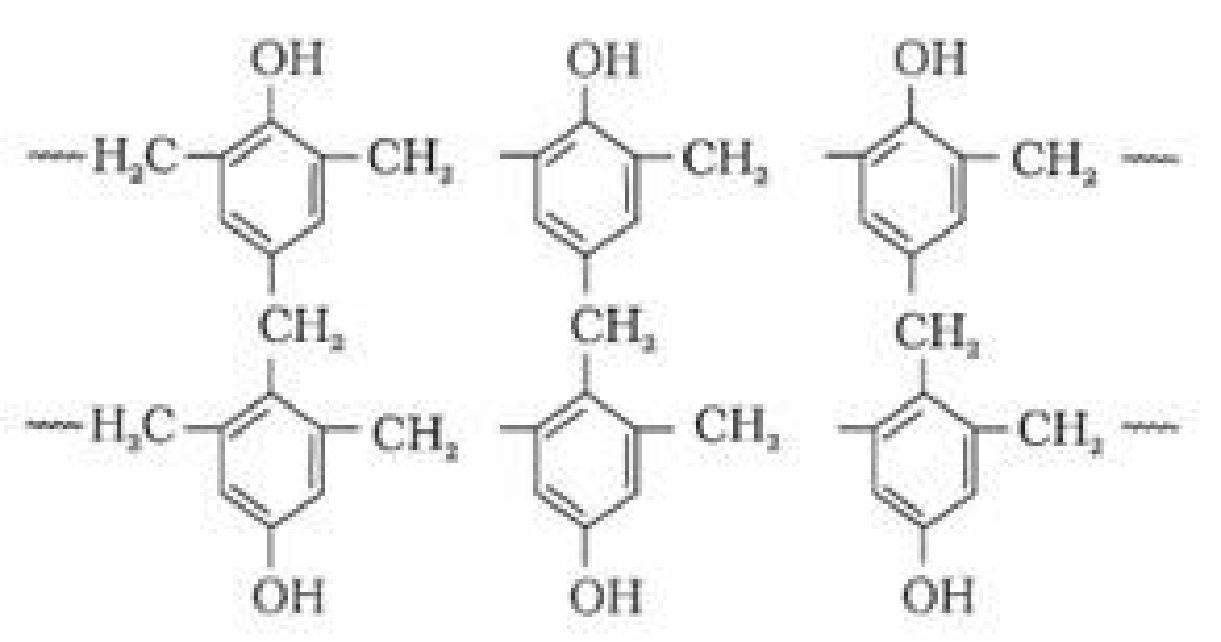
It is used for making combs, phonograph records, electrical switches and handles of various
utensils

It is used in the manufacture of unbreakable crockery.

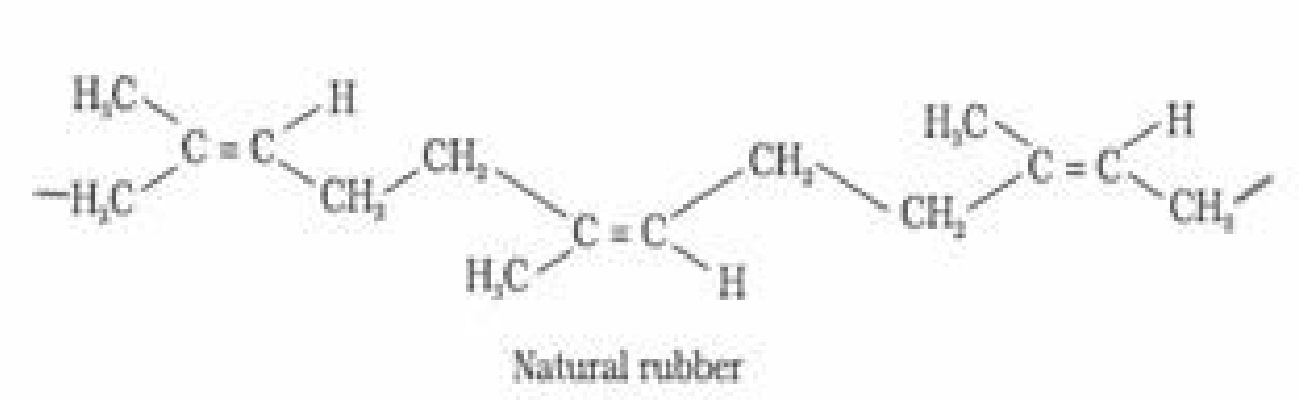
a). Natural rubber: Natural rubber is a linear polymer of isoprene (2-methyl-1, 3-butadiene)
and is also called as cis – 1, 4 – polyisoprene.
b). Synthetic rubber: Synthetic rubbers are either homopolymers of 1, 3 – butadiene
derivatives or copolymers of 1, 3 – butadiene or its derivatives with another unsaturated
monomer.

It is used for manufacturing conveyor belts, gaskets and hoses
B) Buna – N

It is used in making oil seals, tank lining, etc. because it is resistant to the action of petrol,
lubricating oil and organic solvents
C) Buna – S



It is used in speciality packaging, orthopaedic devices and in controlled release of drugs.
b). Nylon 2-nylon 6: It is an alternating polyamide copolymer of glycine(H2N-CH2-COOH)
and amino caproic acid (H2N (CH2)5 COOH)
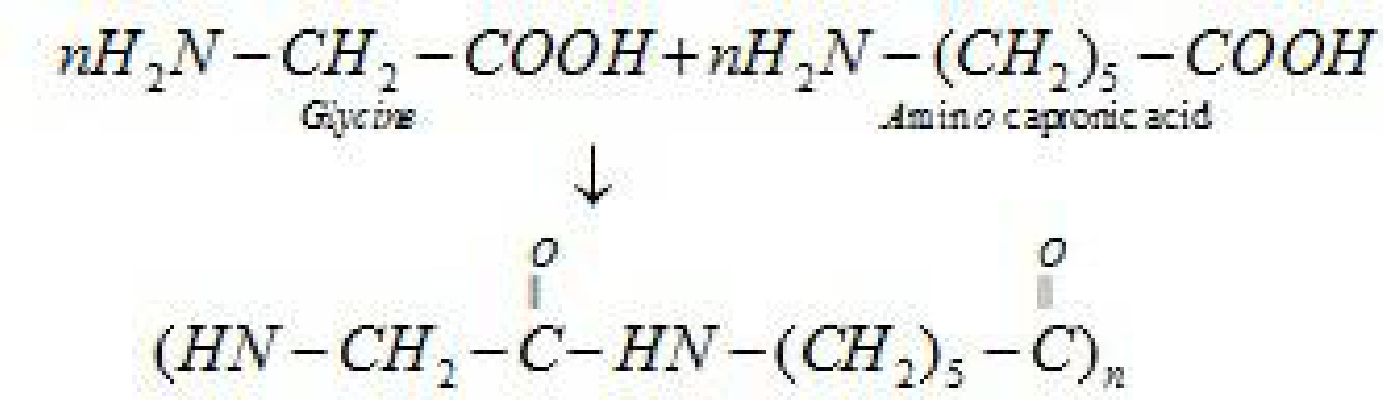
which on heating undergo extensive cross linking in moulds and eventually undergo a
permanent change. Examples – Bakelite, Urea-formaldelyde resins
Condensation Polymerisation or Step Growth polymerization: Polymerisation generally
involves a repetitive condensation reaction between two bi-functional monomers. In
condensation reactions, the product of each step is again a bi-functional species and the
sequence of condensation goes on. Since, each step produces a distinct functionalized species
and is independent of each other, this process is also called as step growth polymerisation.
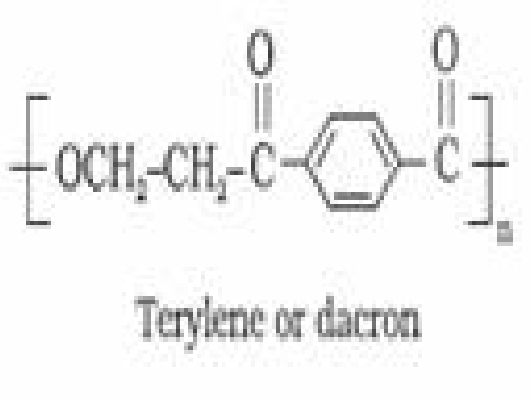
Terylene or Dacron: It is manufactured by heating a mixture of ethylene glycol and
terephthalic acid at 420 to 460 K in the presence of zinc acetate-antimony trioxide catalyst.
Vulcanisation of rubber: The process of heating a mixture of raw rubber with sulphur and
an appropriate additive in a temperature range between 373 K to 415 K to improve upon
physical properties like elasticity, strength etc.
Biodegradable Polymers: Polymers which are degraded by microorganisms within a suitable
period so that biodegradable polymers and their degraded products do not cause any serious
effects on environment.
Examples of biodegradable polymer:
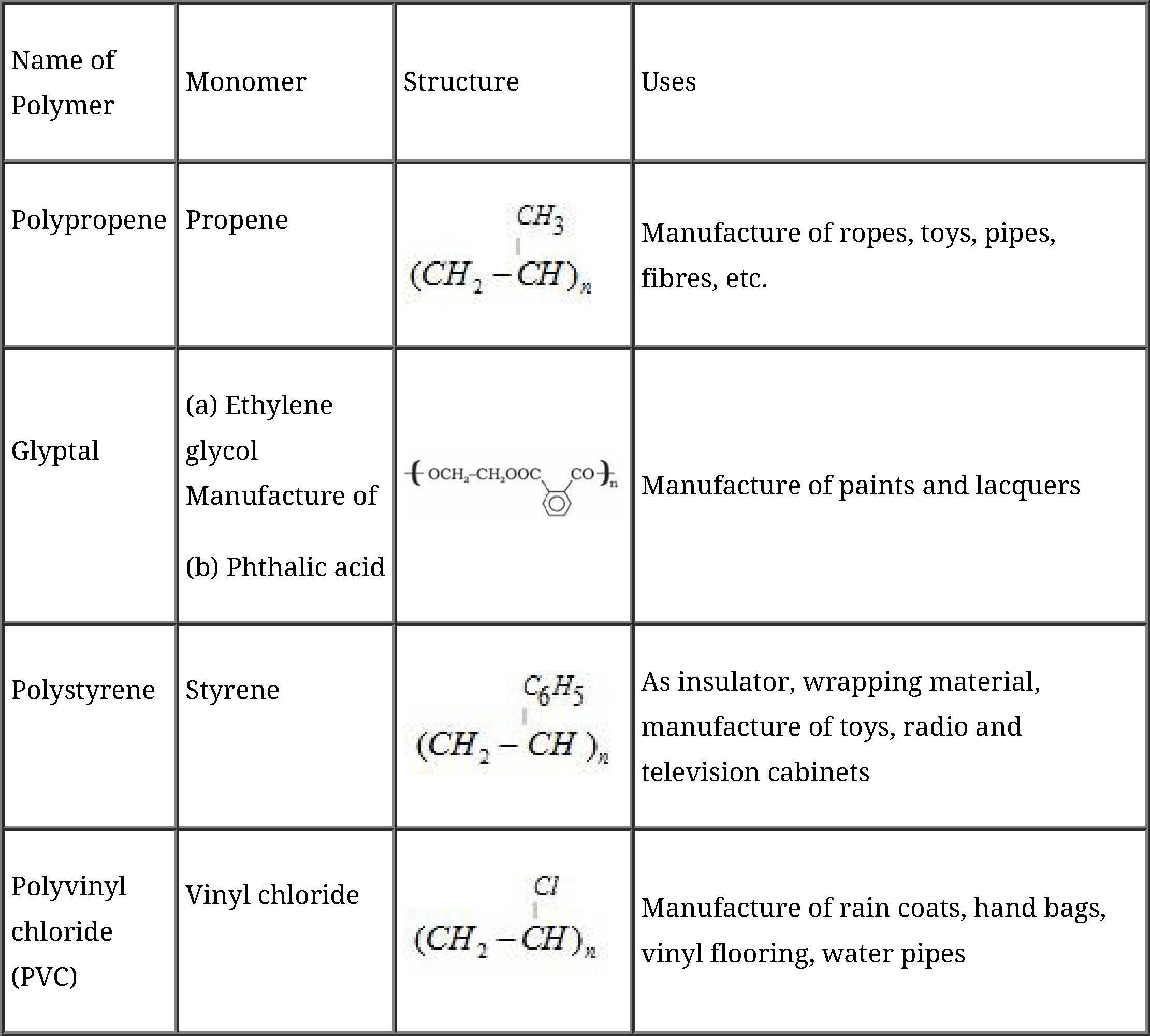
Commercially important polymers along with their structures and uses:
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 14
Biomolecules
Monosaccharides
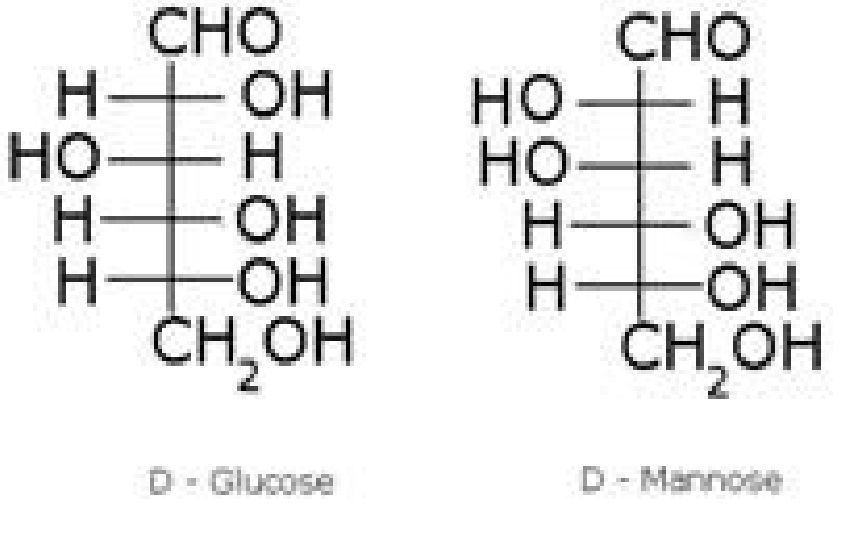
Preparation of glucose (also called dextrose, grape sugar):
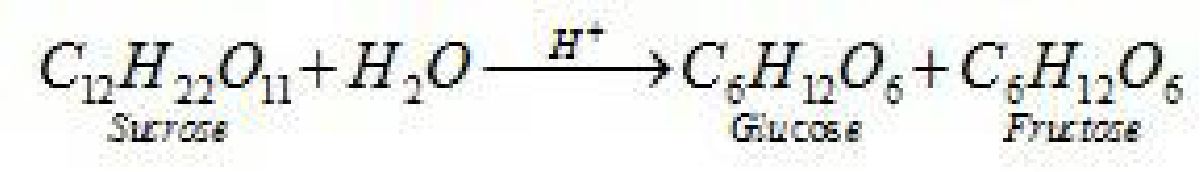
• From starch

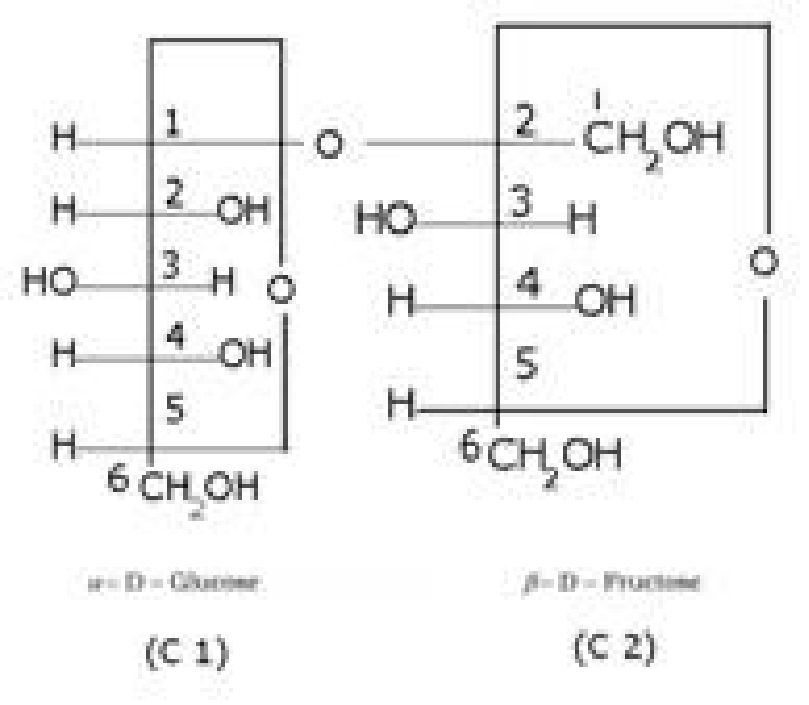
• Structure of glucose
• Structure elucidation of glucose:
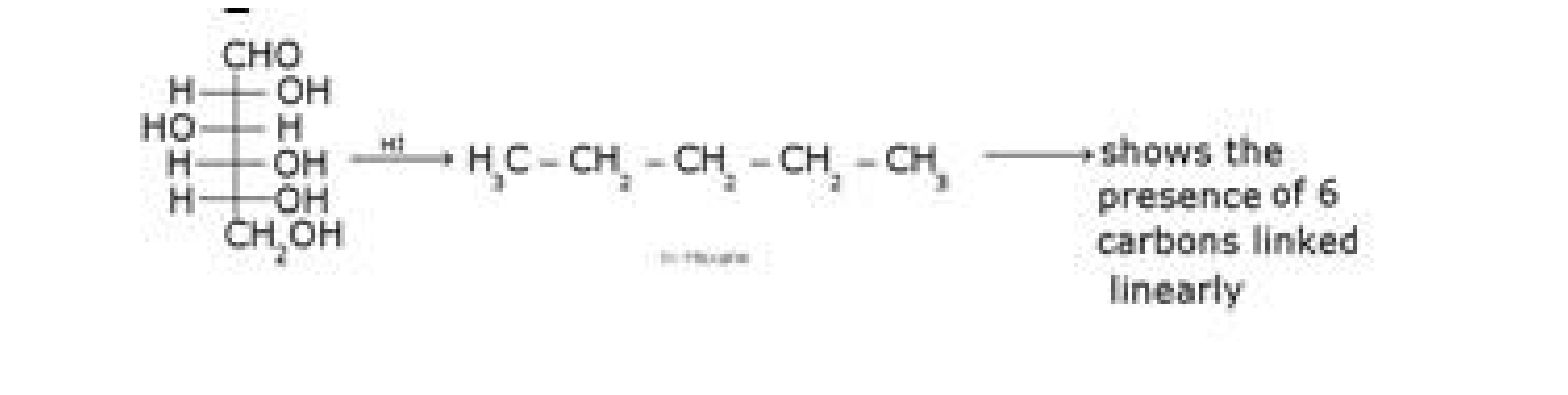
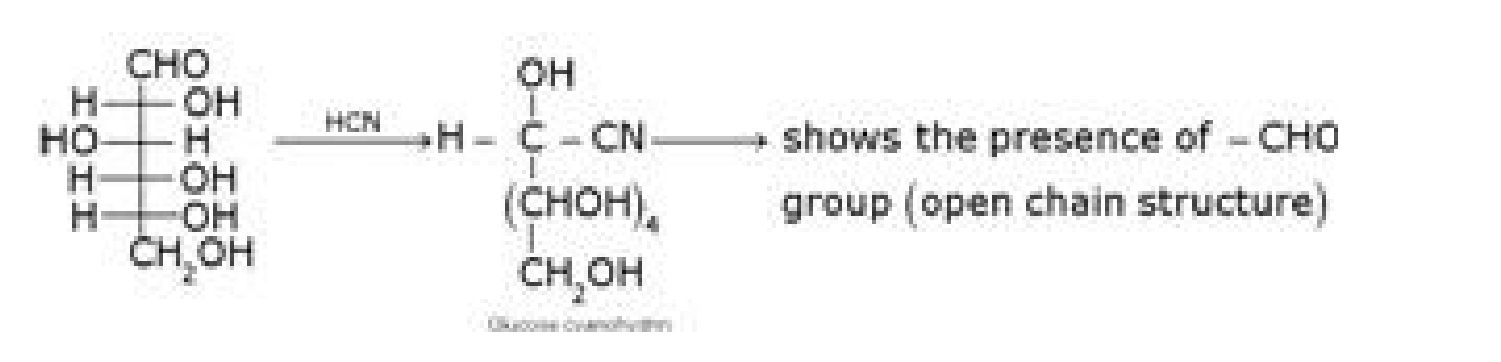
b) D – glucose with HCN
c) D – glucose with NH2OH

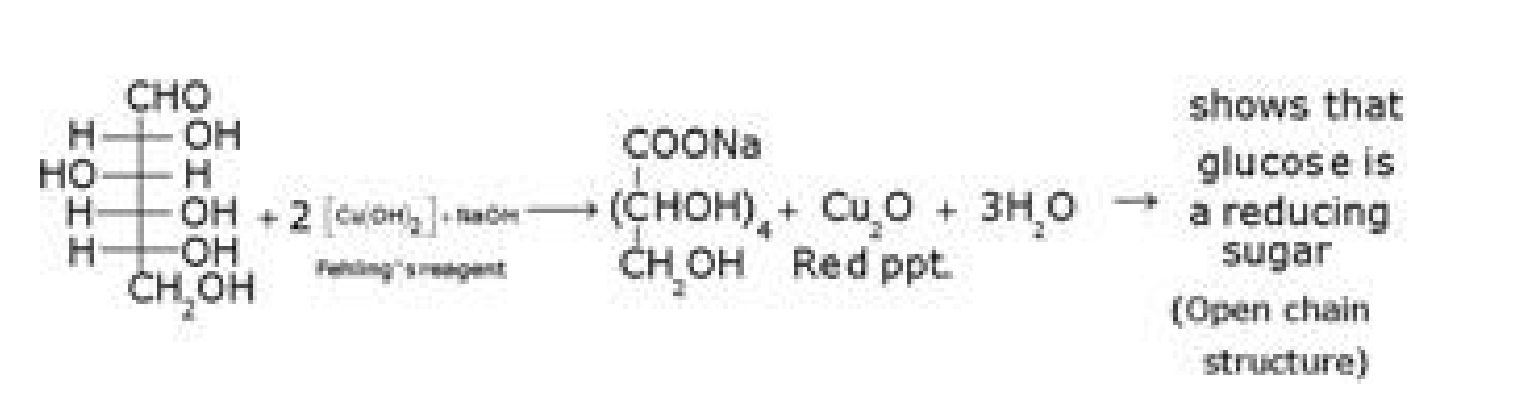
d) D- glucose with Fehling’s reagent
/ 15
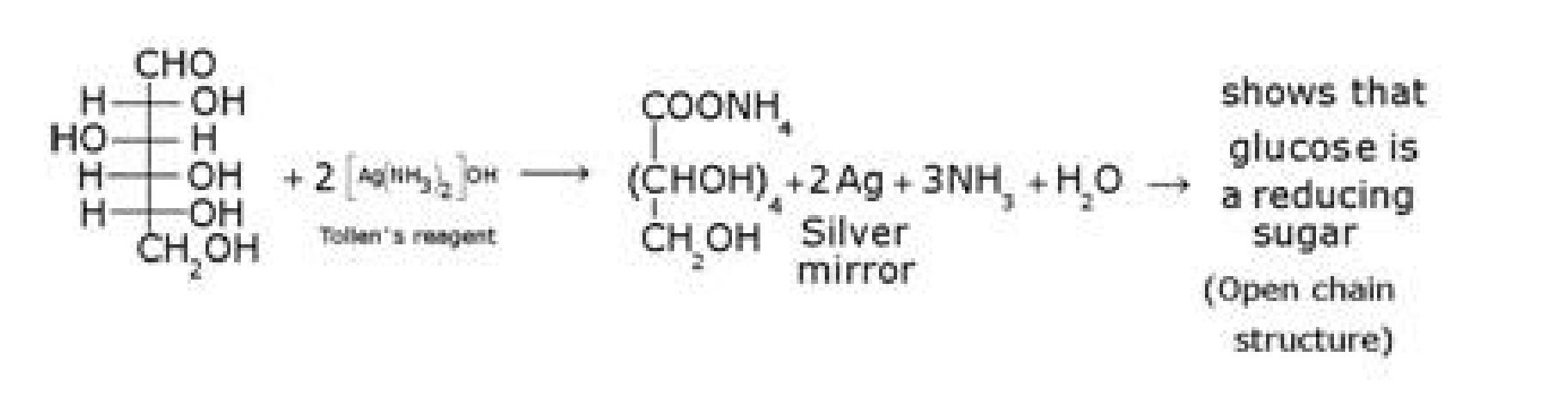
e) D – glucose with Tollen’s reagent
f) D – glucose with nitric acid
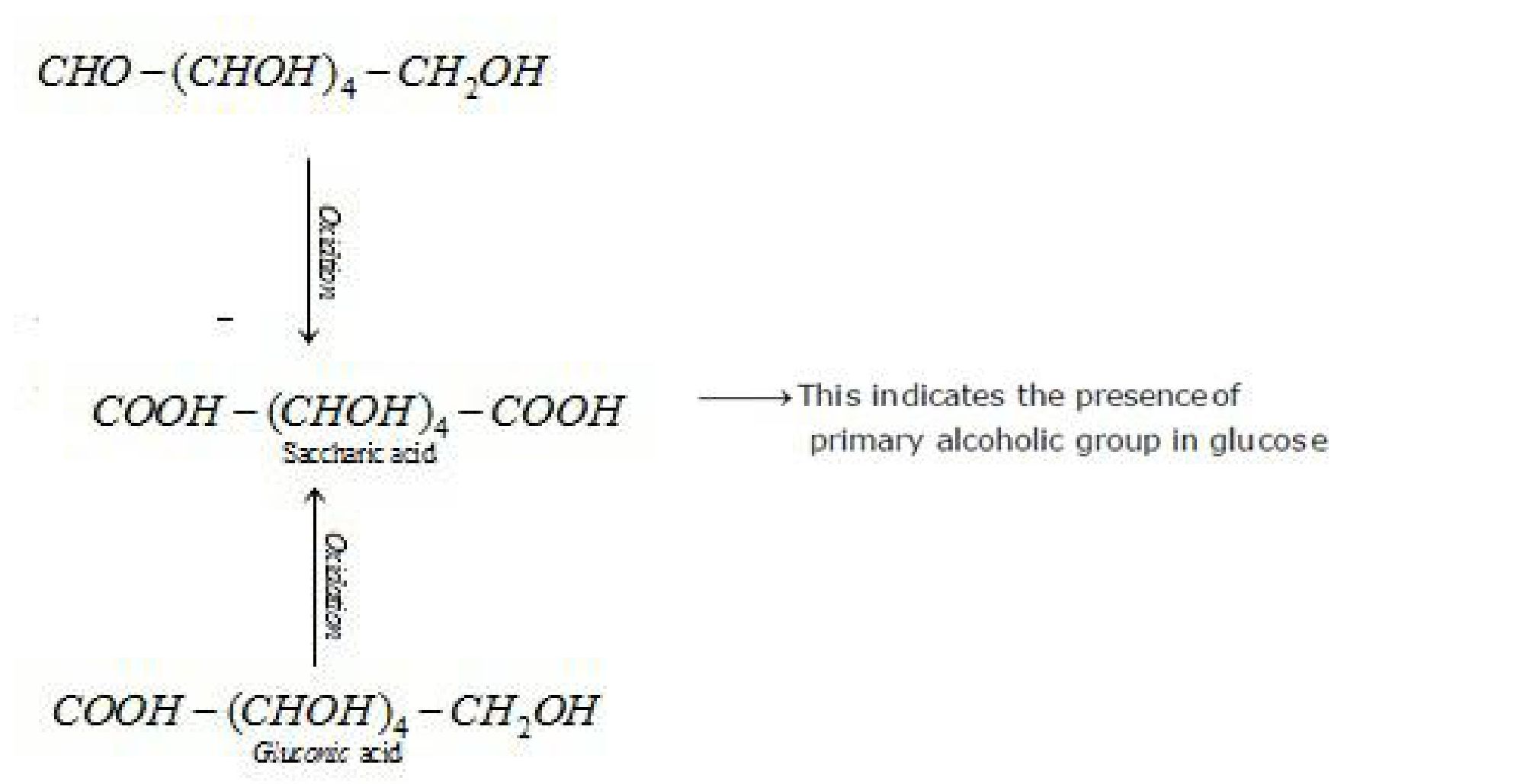
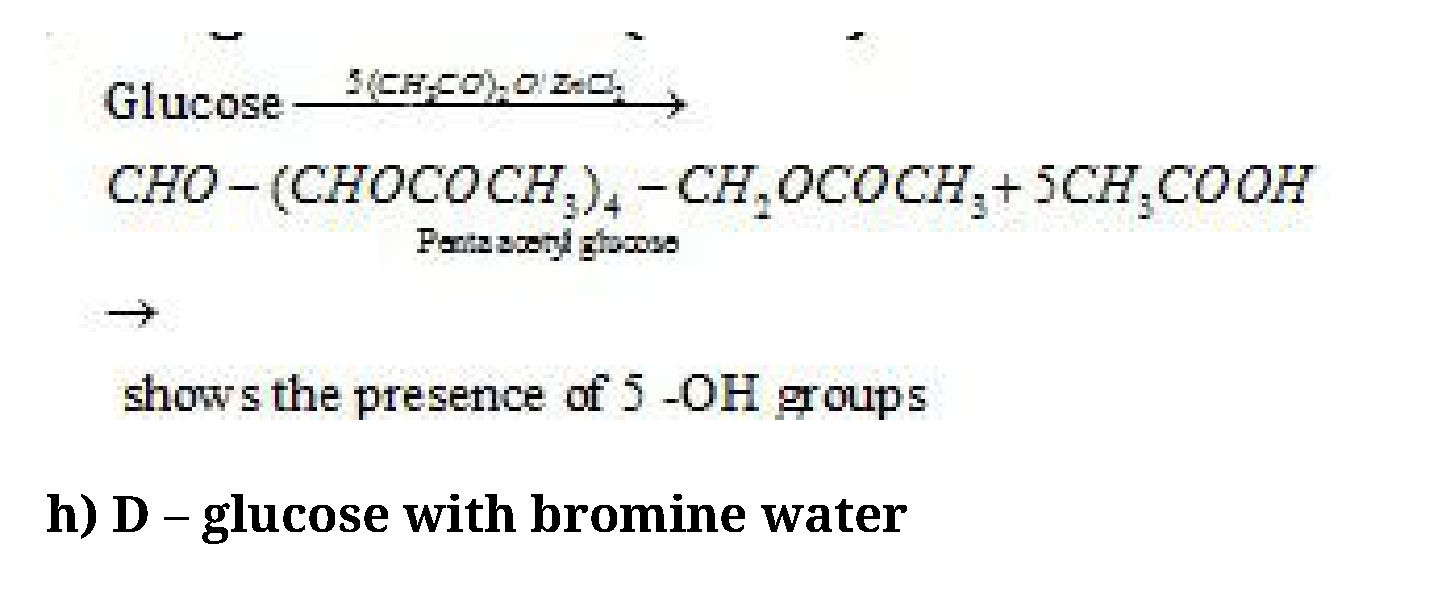
/ 15
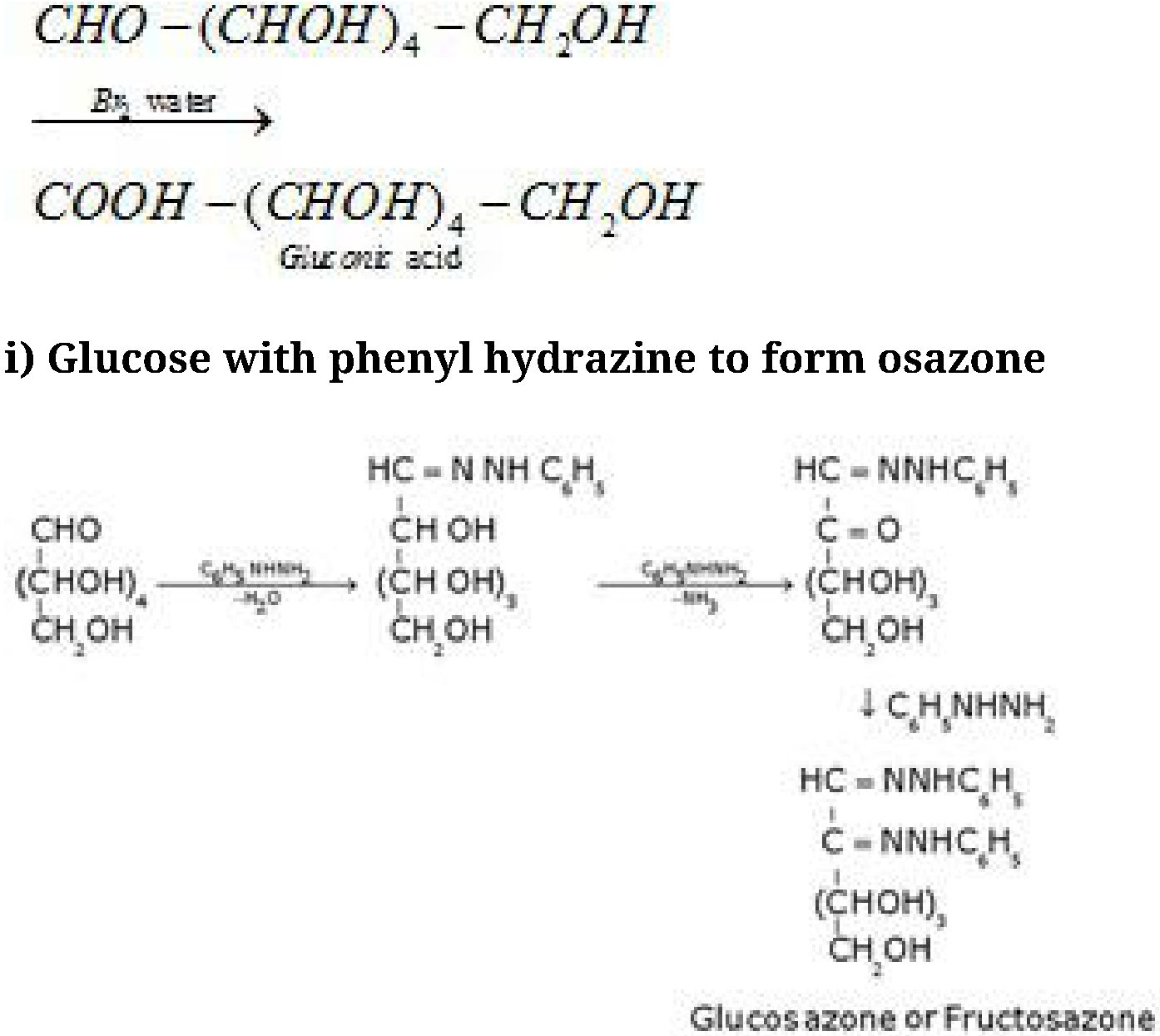
Glucose and fructose gives the same osazone because the reaction takes place at C1 and C2
only.
Other Reactions of Glucose (Presence of ring structure)
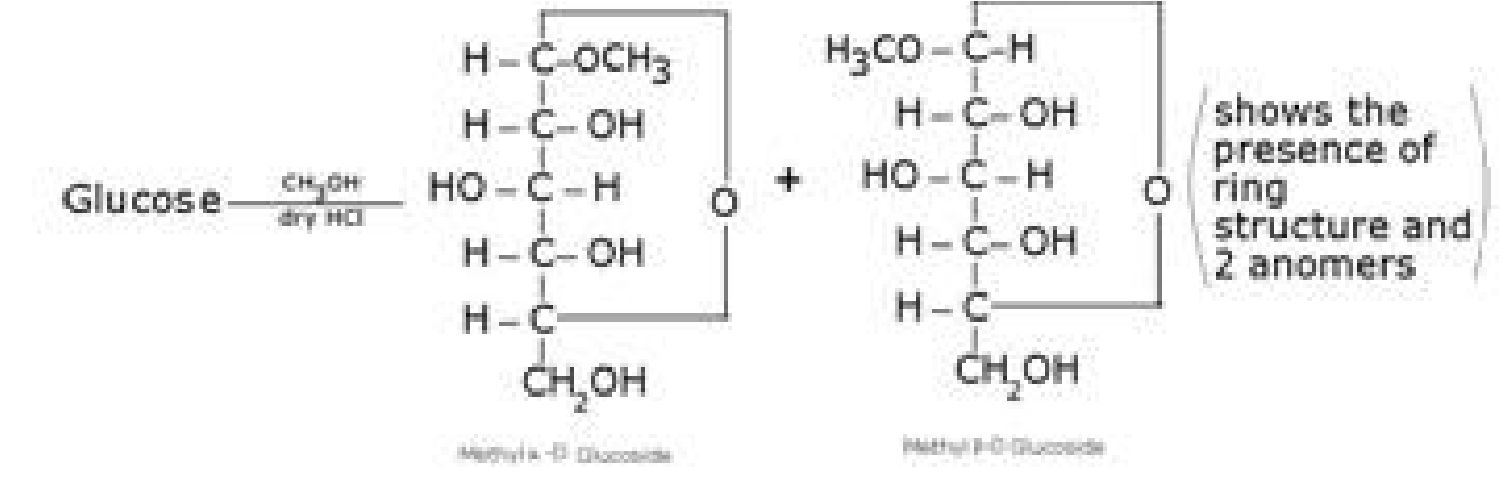
Glucose does not give Schiffs test and does not react with sodium bisulphite and NH3.
Pentaacetyl glucose does not react with hydroxyl amine. This shows the absence of -CHO
group and hence the presence of ring structure.

• Haworth representation of glucose:
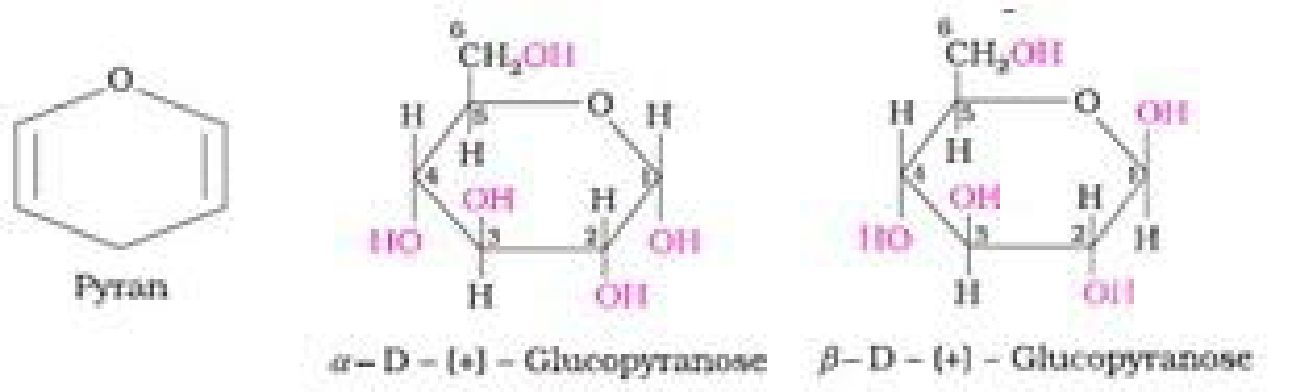
Cyclic structure of fructose:
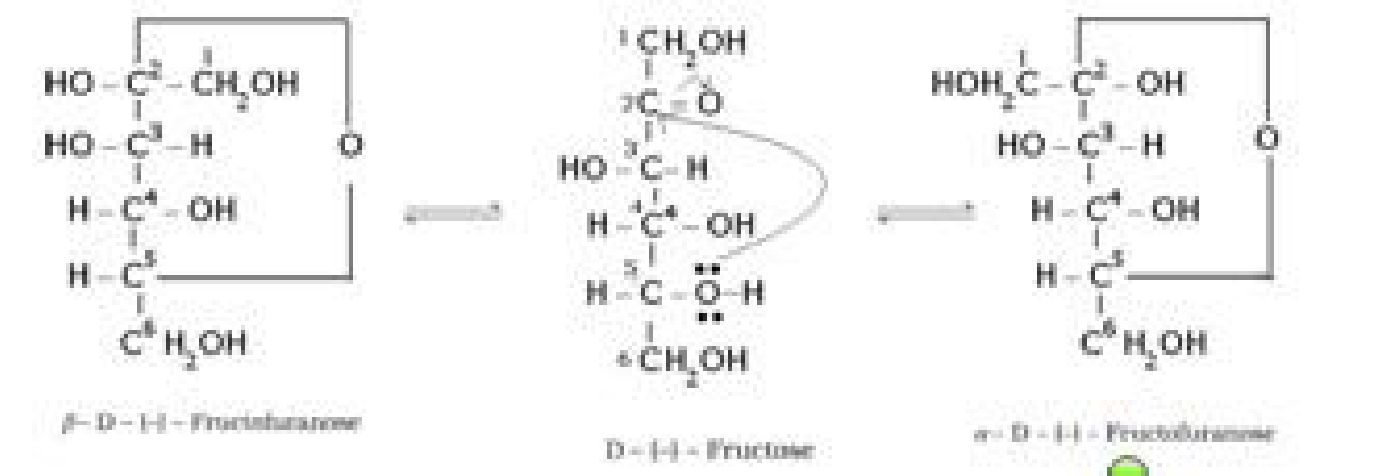
• Haworth representation of fructose
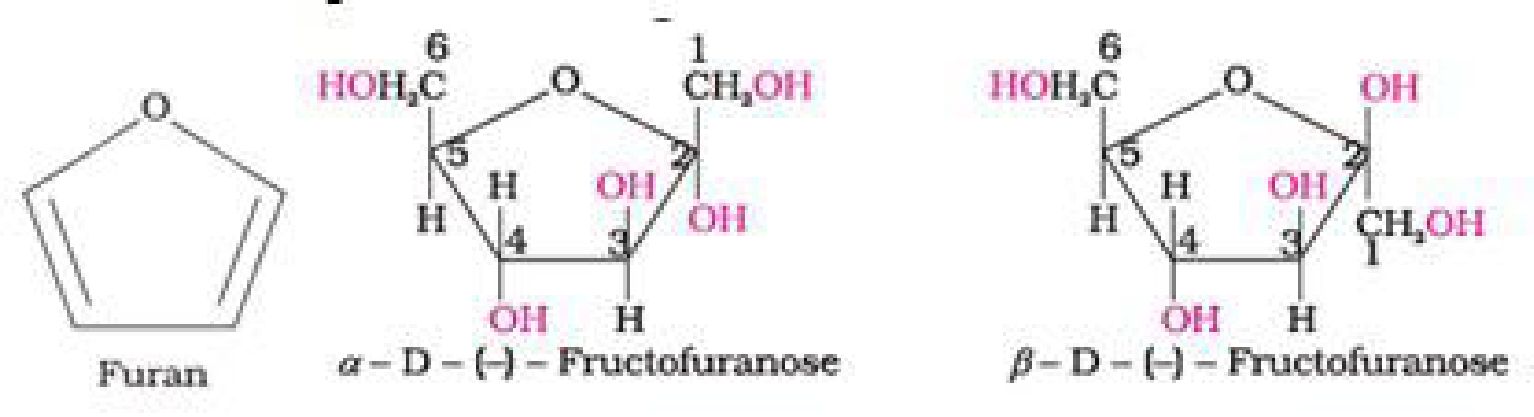
^CiHu°i+C‘Ru0–
D-%izcon D- r>urtare
[*.;.,-+:V^ IafcrJH^
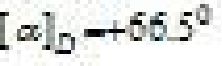
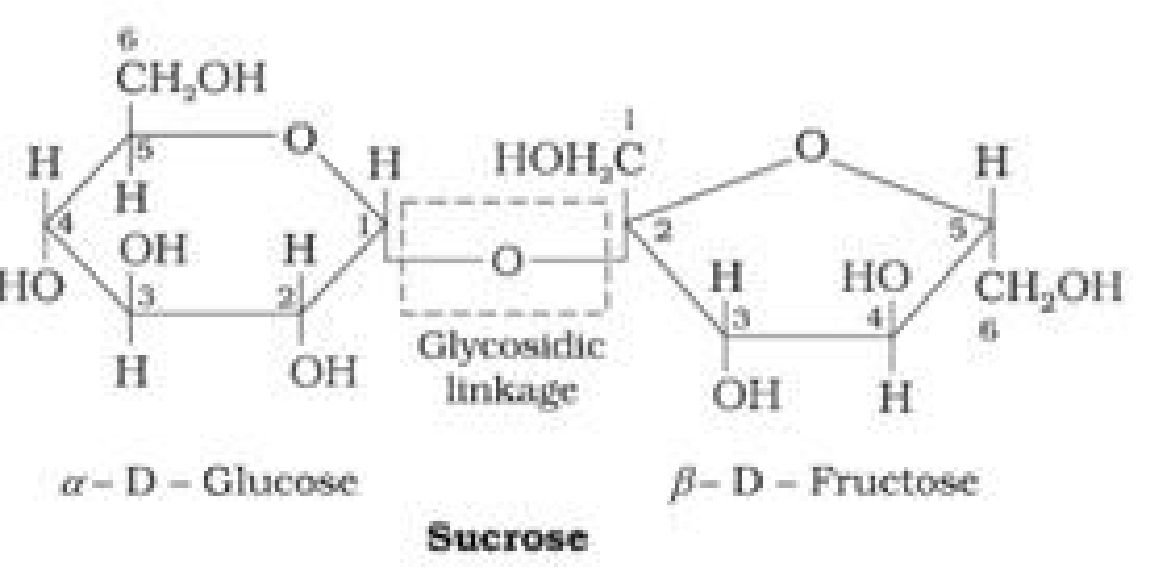
• Haworth Projection of Sucrose:

• Haworth projection of maltose:

• Lactose (Milk sugar):It is composed of p-D-galactose and p-D-glucose. The linkage is
between C1 of galactose and C4 of glucose. Hence it is also a reducing sugar.
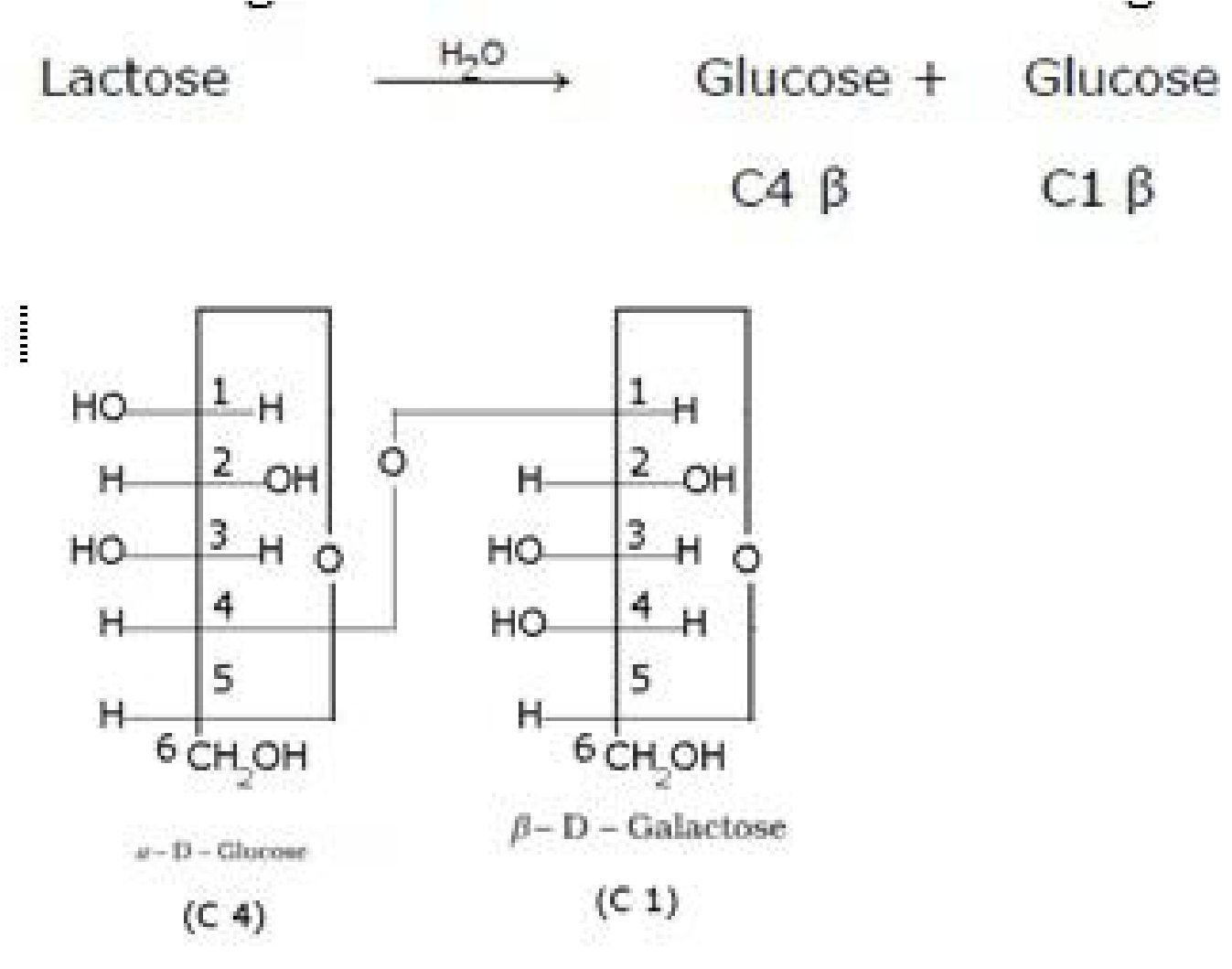
• Haworth projection of lactose:

Amino acids contain amino (-NH2) and carboxyl (-COOH) functional groups.
L
Where R – Any side chain
Most naturally occurring amino acids have L – Config.
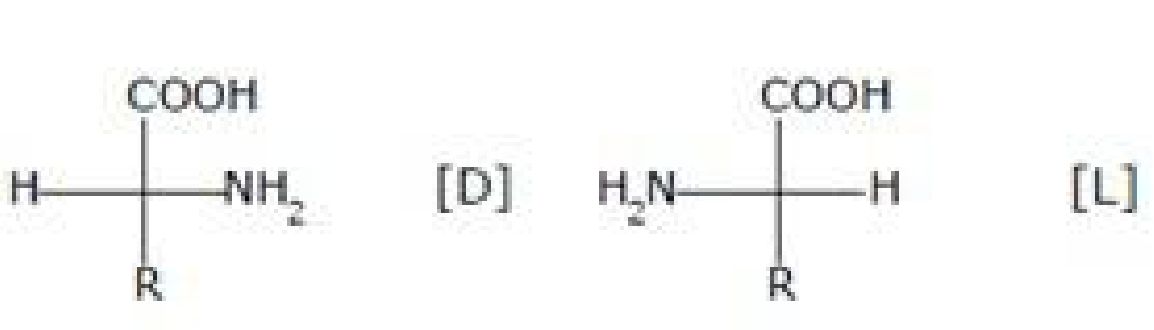
• Types of amino acids:
a). Essential amino acids: The amino acids which cannot be synthesised in the body and
must be obtained through diet, are known as essential amino acids. Examples: Valine,
Leucine
• Zwitter ion form of amino acids:
O O
R-CH-C-O-H^R- CH-C-O-
jffiR W1
2 j
■JSi iiii^ ioa^
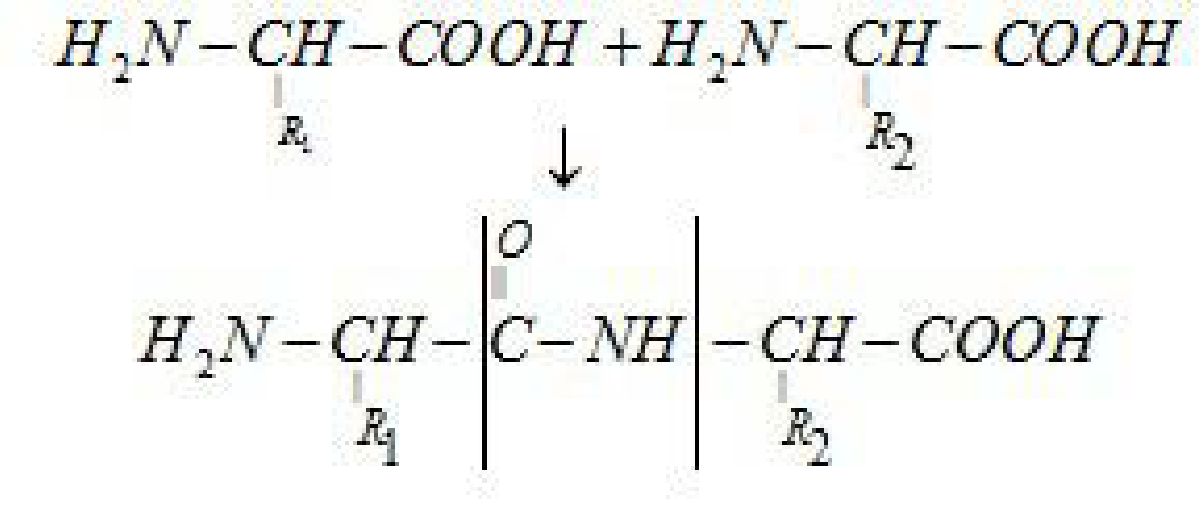
Peptide link age
a- Helix:
and a 13 – membered ring is formed by H – bonding.
/3- pleated sheet:
1. Base + sugar
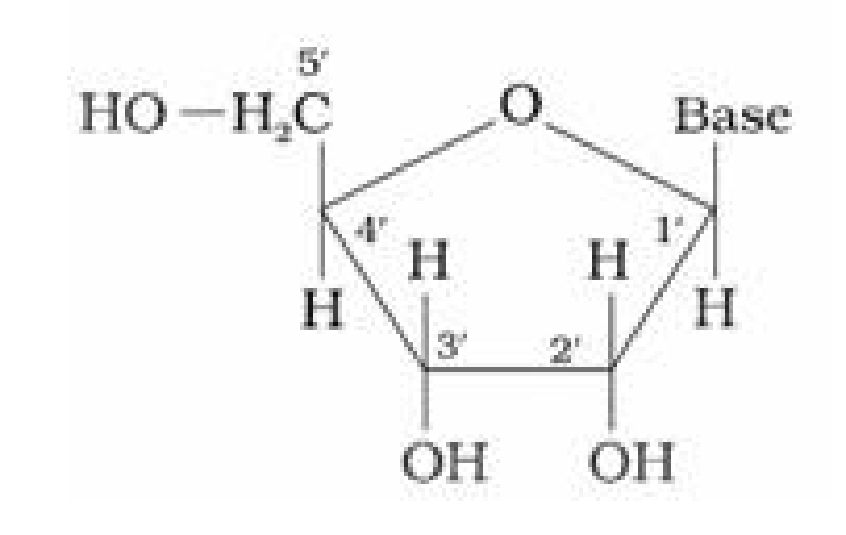
1. Base + sugar + phosphate group

formed between specific pairs of bases.
• Important vitamins, their sources and their deficiency diseases:
|
Name of |
Sources |
Deficiency diseases |
|
Vitamin A |
Fish liver oil, |
xerophthalmia |
|
Vitamin B1 |
Yeast, milk, green |
Beriberi (loss of appetite, retarded growth) |
|
Vitamin B2 |
Milk, egg white, liver, |
Cheilosis (fissuring at corners of mouth and lips), digestive |
|
Vitamin B6 |
Yeast, milk, egg yolk, |
Convulsions |
|
Vitamin B12 |
Meat, fish, egg and curd |
Pernicious anaemia (RBC deficient in haemoglobin) |
|
Vitamin C |
Citrus fruits, amla and |
Scurvy (bleeding gums) |
|
Vitamin D |
Exposure to sunlight, fish |
Rickets (bone deformities in children) and (soft bones and joint pain in adults) |
|
Vitamin E |
Vegetable oils like wheat |
Increased fragility of RBCs and |
|
Vitamin K |
Green leafy vegetables |
Increased blood clotting time |
• Maltose:
Maltose is composed of two a-D-glucose units in which C1 of one glucose (I) is linked to C4
of another glucose unit (II).
The free aldehyde group can be produced at C1 of second glucose in solution and it shows
reducing properties so it is a reducing sugar.
Sucrose (invert sugar): ↑
CBSE Class-12 Chemistry
Quick Revision Notes
Chapter 13
Amines
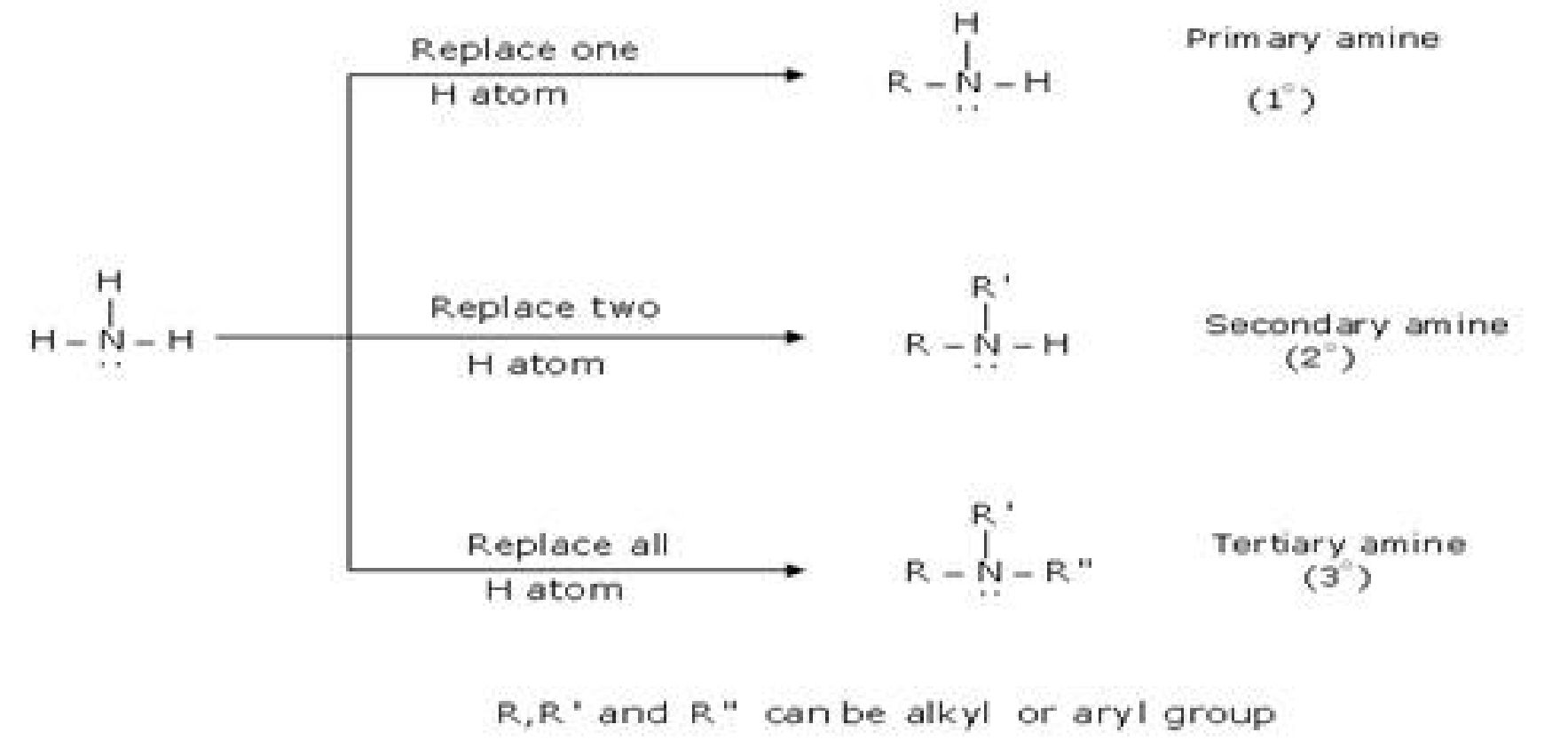
• Amines: Amines are regarded as derivatives of ammonia in which one, two or all
three hydrogen atoms are replaced by alkyl or aryl group.
• Classification of amines:
(i) By reduction of nitro compounds: Nitro compounds can be catalytically reduced by
passing hydrogen gas in presence of Raney Ni, finely divided Pt or Pd as catalyst at room
temperature.
Ni,Pt or pd
a)
Ni, Pt or pd
b)
Nitro compounds can also be reduced with active metals such as Fe, Sn, Zn etc. with conc.
HCl.
Sn/HCl or Fe/HCl
a)
Sn/HCl or Fe/HCl
(ii) By Hoffmann’s method (Ammonolysis of alkyl halides): Reaction of alkyl halides with
an ethanolic solution of ammonia in a sealed tube at 373 K forms a mixture of primary,
secondary and tertiary amine and finally quarternary ammonium salt. Process of cleavage of
C-X bond by ammonia is called ammonolysis.
+■ —
RNH2 — > R2NH f–l: > R3N K>: > S4 A7 X
(£) (2e) (je) Q*at9T#nrj/.
3Tjy.PT.LUTL E-L |[ I
NaOH
a)
(l°a min e)
NaOH
b)
(2°amine)
NaOH
c)
(3° a min e)
Method is not suitable for preparation of aryl amines because aryl amines are relatively less
reactive than alkyl halides towards nucleophilic substitution reactions.
H2/Ni
Or
Na(Hg)/C2H5OH
Or
LiAlHt
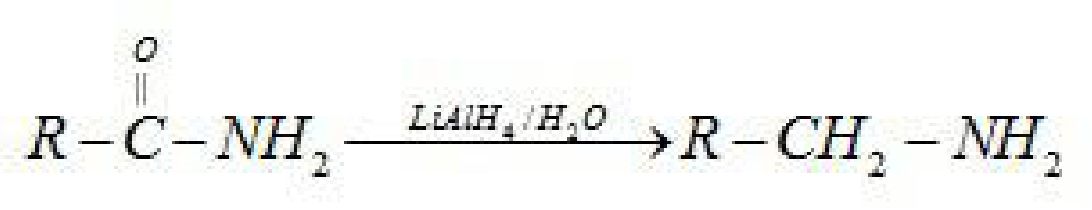

Aromatic primary amines cannot be prepared by this method because aryl halides do not
undergo nucleophilic substitution with potassium phthalimide.
parent amide.
0
Il
i
R – NH2 + Na2 CO2 + 2 NaBr + 2 H2O
Physical properties of amines:
• Chemical properties of amines:
[.R-NHz][OH]
[R-NH2][H20\
[R-NHs][OH]
Or K[H20] =
[R-NH2]
_ [R-NH3][OH]
b [R-NH2]
pKb = -Iogif6
Greater Kb value or smaller pKb indicates base is strong.
(i) The order of basicity of amines in the gaseous phase follows the expected order on the
basis of +I effect: tertiary amine > secondary amine > primary amine > NH3
(ii) In aqueous solution it is observed that tertiary amines are less basic than either primary
or secondary amines. This can be explained on basis of following factors:
On the basis of solvation effect order of basicity of aliphatic amines should be primary
amine>secondary amine>tertiary amine.
this case order of basicity in aqueous medium is
(CH3)2NH > CH3NH2 > (CH3)3N > NH3
When alkyl group is ethyl group order of basicity in aqueous medium is
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3
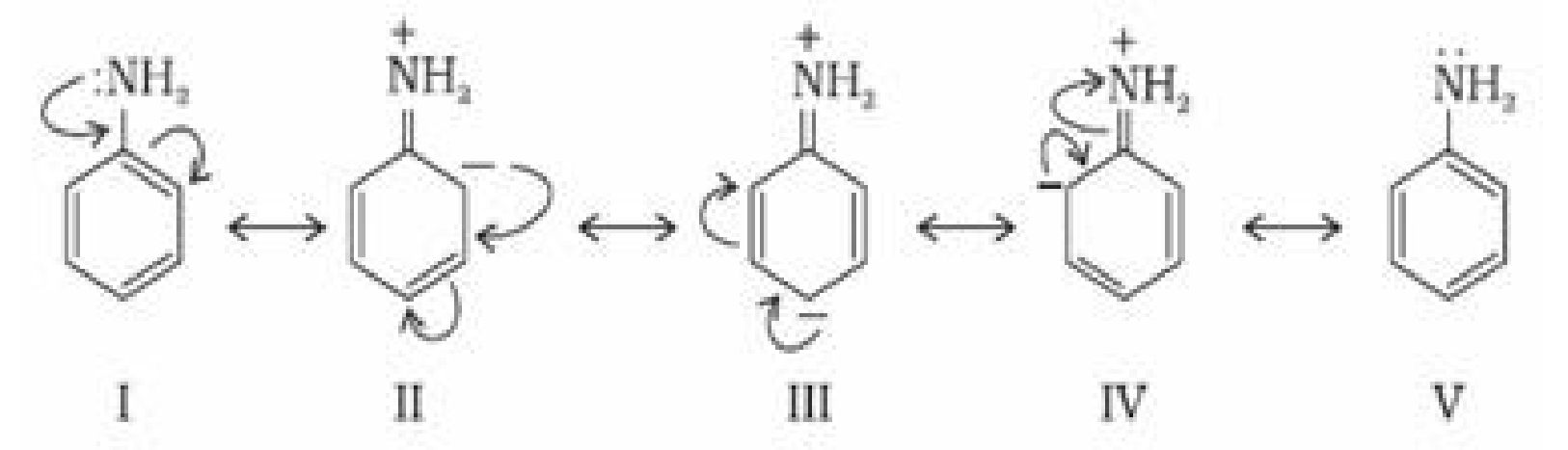
In the above resonating structures there is a positive charge on nitrogen atom making the
lone pair less available for protonation. Hence aniline is less basic than ethyl amine which
has no resonating structures. Less basicity of aniline can also be explained by comparing the
relative stability of aniline and anilinium ion obtained by accepting a proton. Greater the
number of resonating structures, greater is the stability of that species.
Aniline is resonance hybrid of five resonating structures whereas anilinium ion has only two
resonating structures.
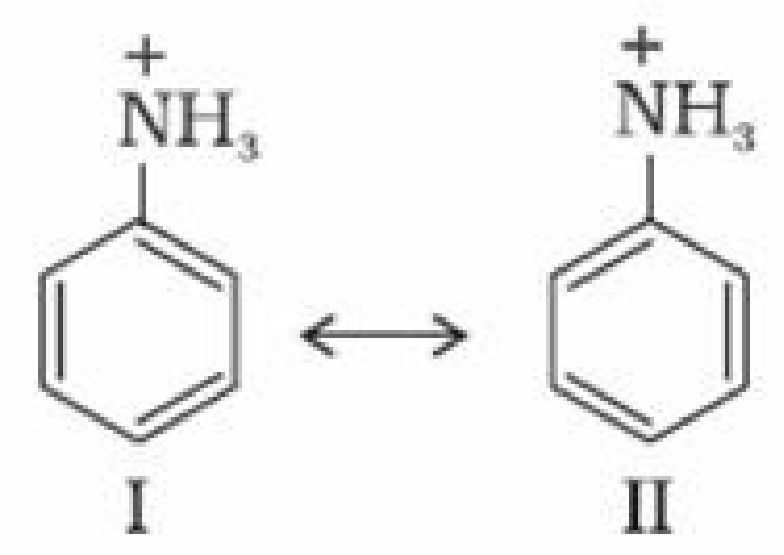
Thus aniline has less tendency to accept a proton to form anilinium ion.
a) Acylation Reaction: Aliphatic and aromatic primary and secondary amines (which
contain replaceable hydrogen atoms) react with acid chlorides, anhydrides and esters to
form substituted amide. Process of introducing an acyl group (R-CO-) into the molecule is
called acylation. The reaction is carried out in the presence of a stronger base than the
amine, like pyridine, which removes HCl formed and shifts the equilibrium to the product
side.
Base
R – NH2 + RCOCl > RNHCOR + HCl
Add chloride Substituted amide
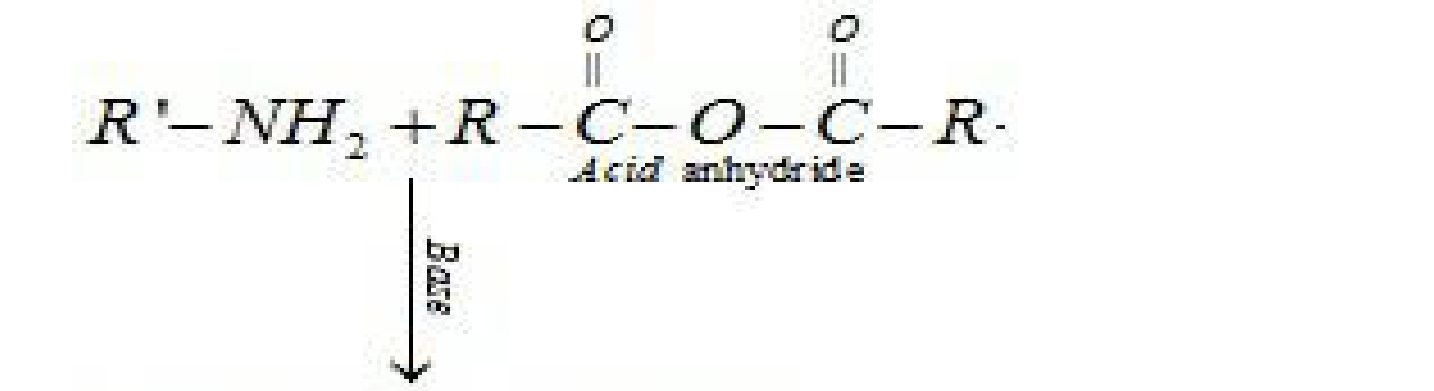
^Li bzmwed em^
Since tertiary amine do not contain replaceable hydrogen atom they do not undergo
acylation reaction.
b) Carbylamine reaction: Only aliphatic and aromatic primary amines on heating with
chloroform and ethanolic potassium hydroxide form isocyanides or carbylamines.
R – NH0 + CHCl + 3 KOH
2. .-
|
1 F
R – NC + 3KCl + ^H2O
Secondary and tertiary amines do not give the above test.
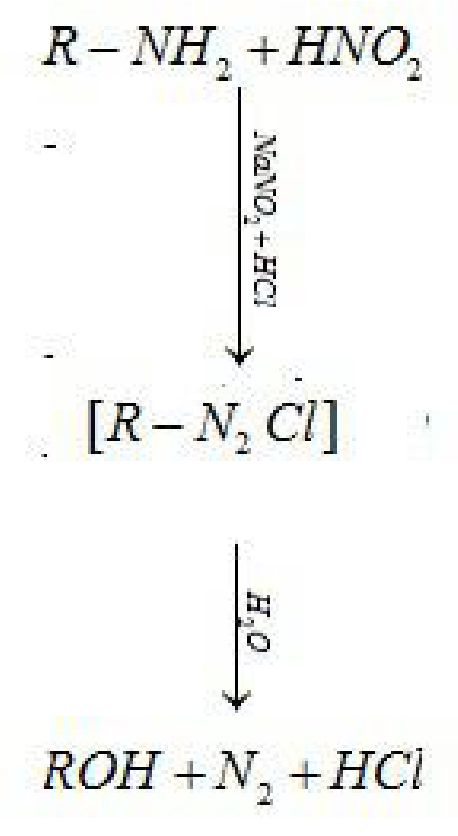
(i) Primary aliphatic amine on reaction with nitrous acid (HNO2) forms aliphatic
diazoniumsalt which decomposes to form alcohol and evolve nitrogen.
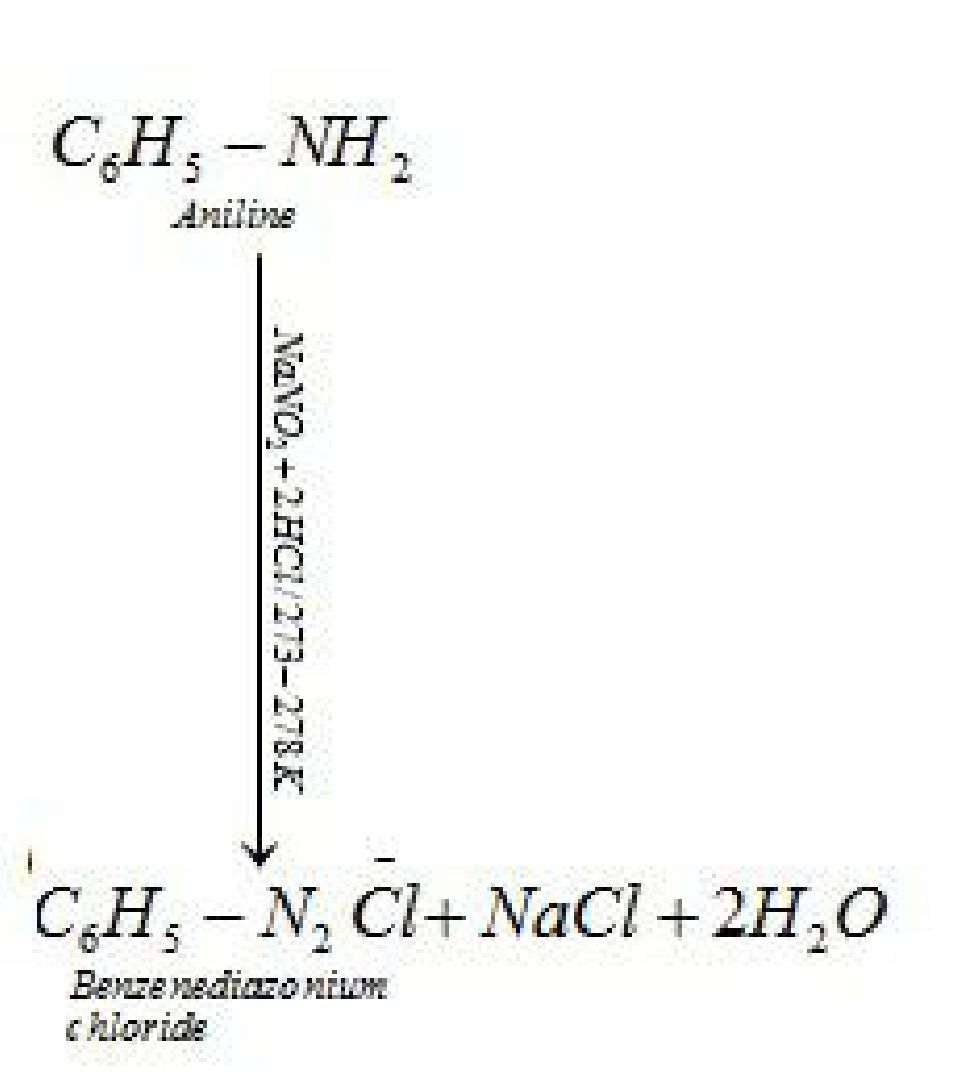
(ii) Primary aromatic amines react with nitrous acid (HNO2) in cold (273-278 K) to form
diazonium salt.
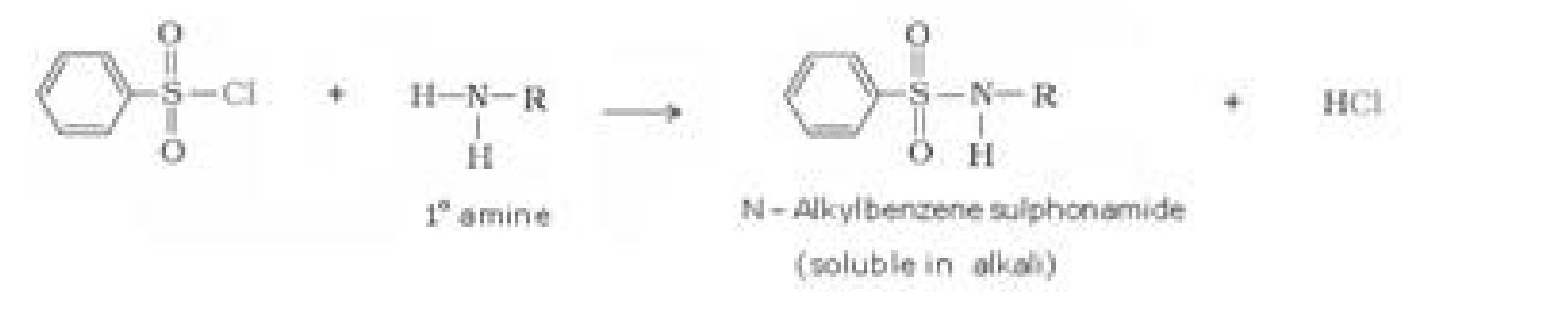
The hydrogen attached to nitrogen in sulphonamide formed by primary amine is strongly
acidic due to the presence of strong electron withdrawing sulphonyl group. Hence, it is
soluble in alkali.

Since sulphonamide formed by secondary amine does not contain any hydrogen atom
attached to nitrogen atom, so it is not acidic. Hence it is insoluble in alkali.
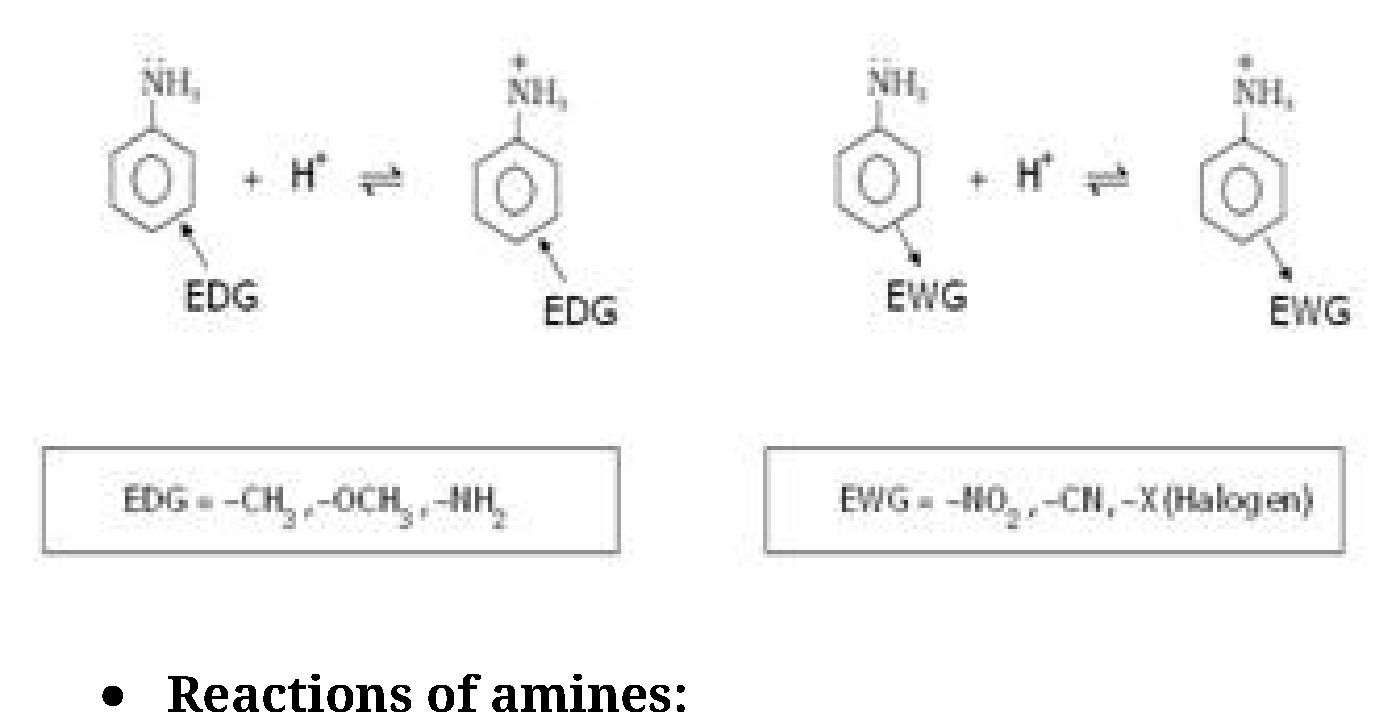
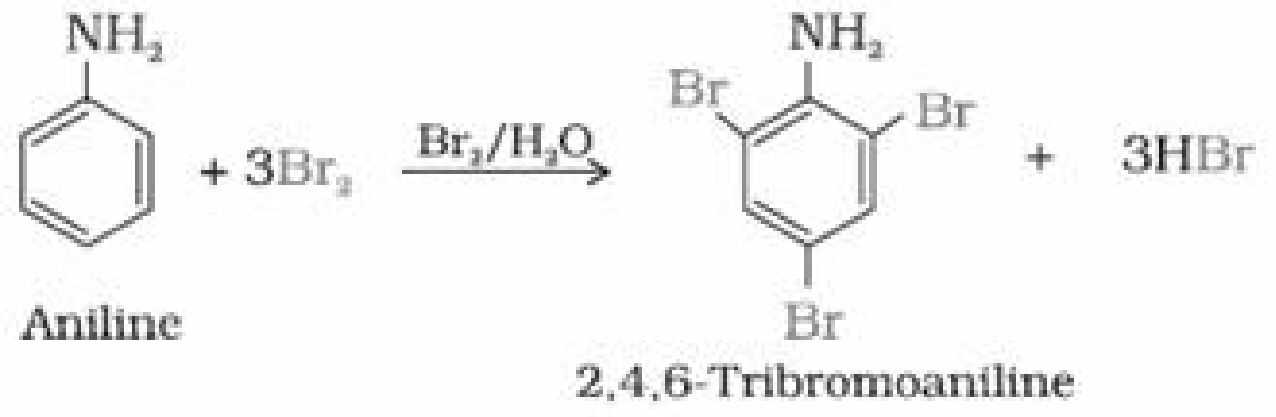
• Ring substitution in aromatic amine: Aniline is more reactive than benzeneand
undergoes electrophilic substitution reaction preferably at ortho and para position.
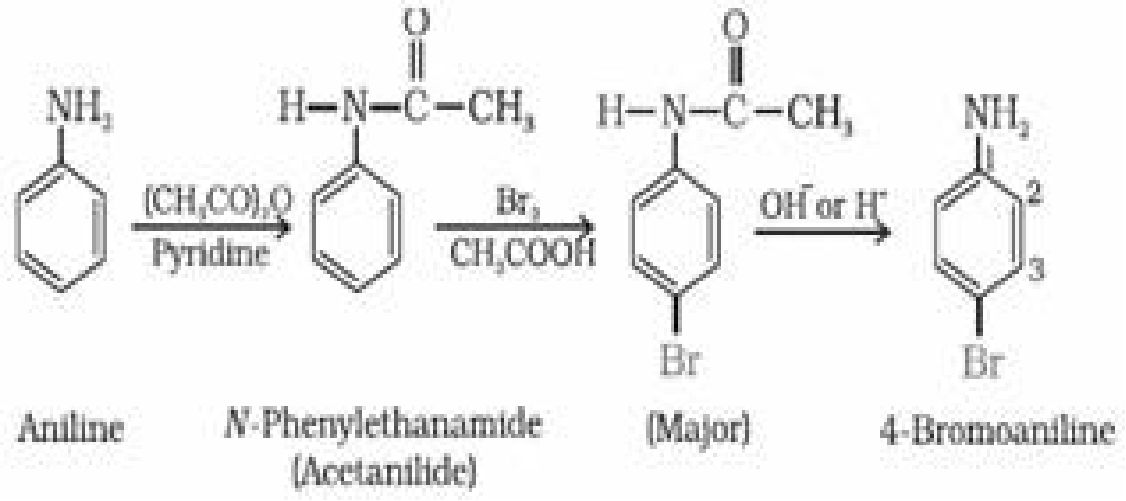
(i) Bromination: Aniline reacts with bromine water at room temperature to give a white
precipitate of 2, 4, 6-tribromoaniline
In order to stop reaction at monosubstitution activating effect of -NH2 group is reduced by
acetylation. This prevents di and tri substituted products. Acetyl group is removed by
hydrolysis.
(a) Under strongly acidic medium aniline gets protonated to form anilinium ion, which is
deactivating group and is meta directing. Hence minitroaniline is also formed in 47% along
with ortho and para products.
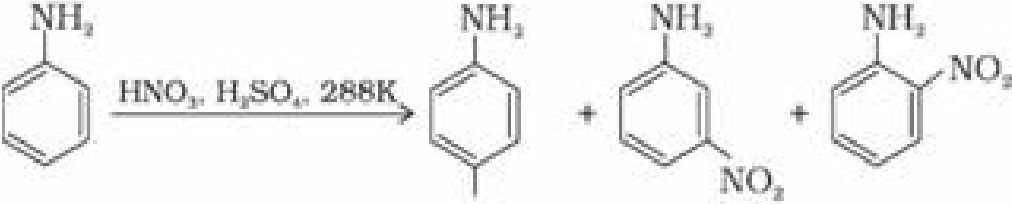
NOj
(51¾) (47%) (2%)
Aromatic amines cannot be nitrated directly because HNO3 being a strong oxidising agent
oxidises it forming black mass.
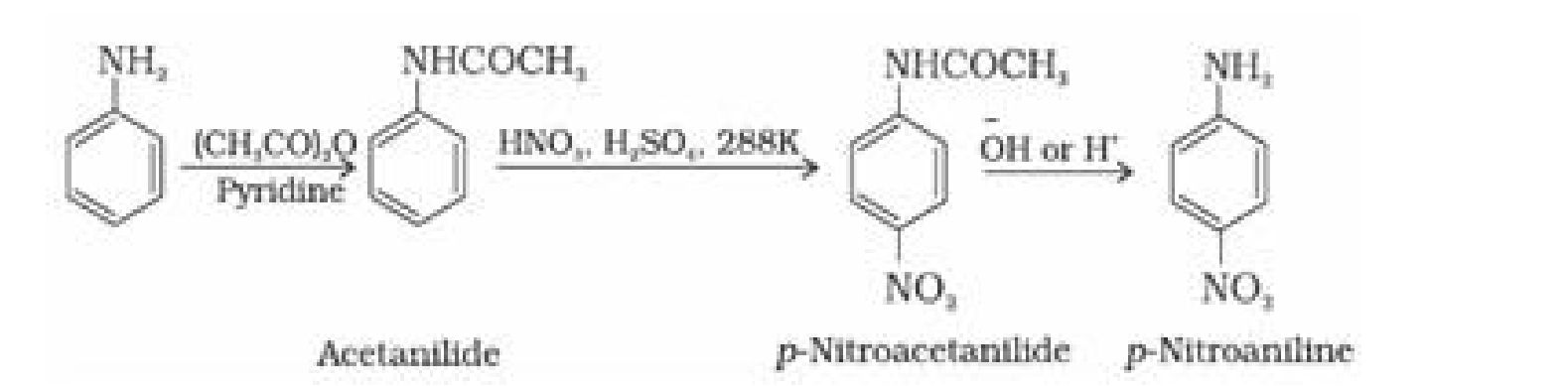
(b) Nitration by protecting the -NH2 group by acetylation reaction with acetic anhydride:
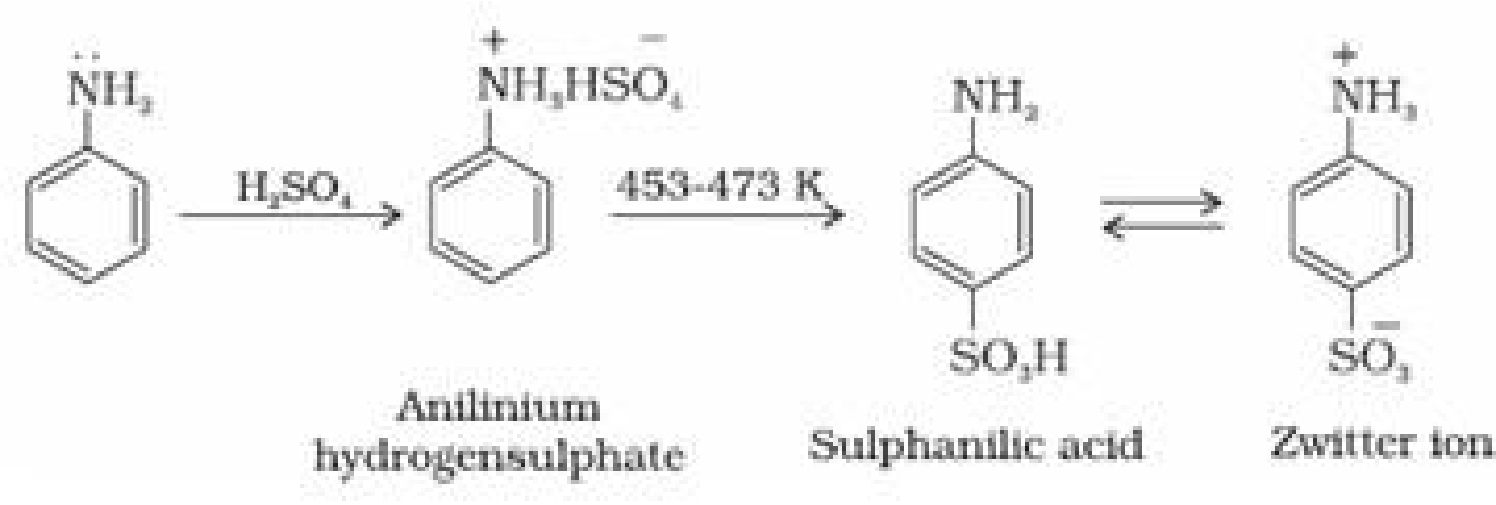
iii) Sulphonation: Aniline reacts with conc. H2SO4 to form aniliniumhydrogensulphate which
on heating with sulphuric acid at 453-473K produces p-aminobenzenesulphonic acid,
commonly known as sulphanilic acid, as the major product.
• Reactions ofbenzene diazonium chloride:
a) Reactions involving displacement of nitrogen:
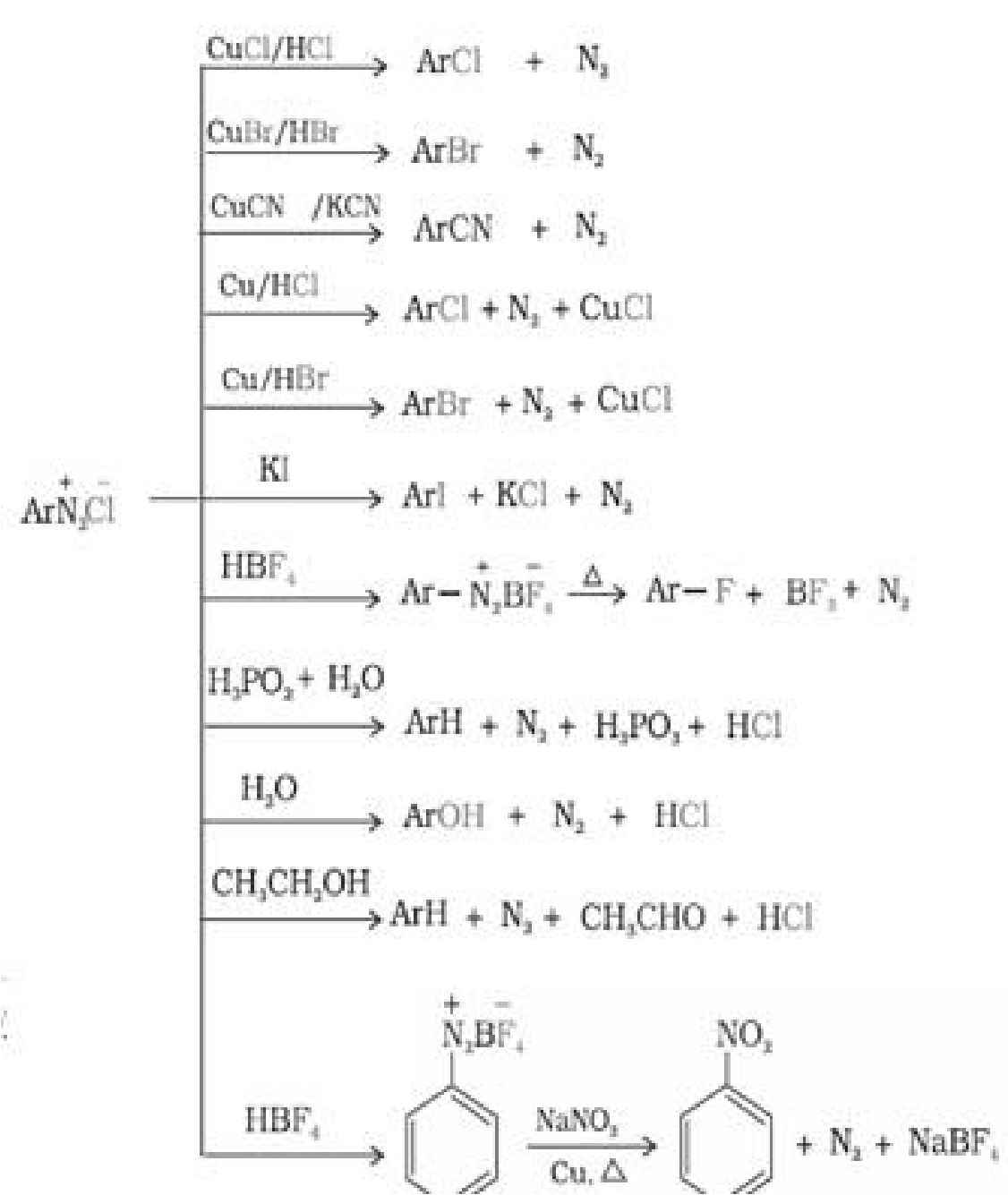
Material Downloaded From SUPERCOP
/ 11
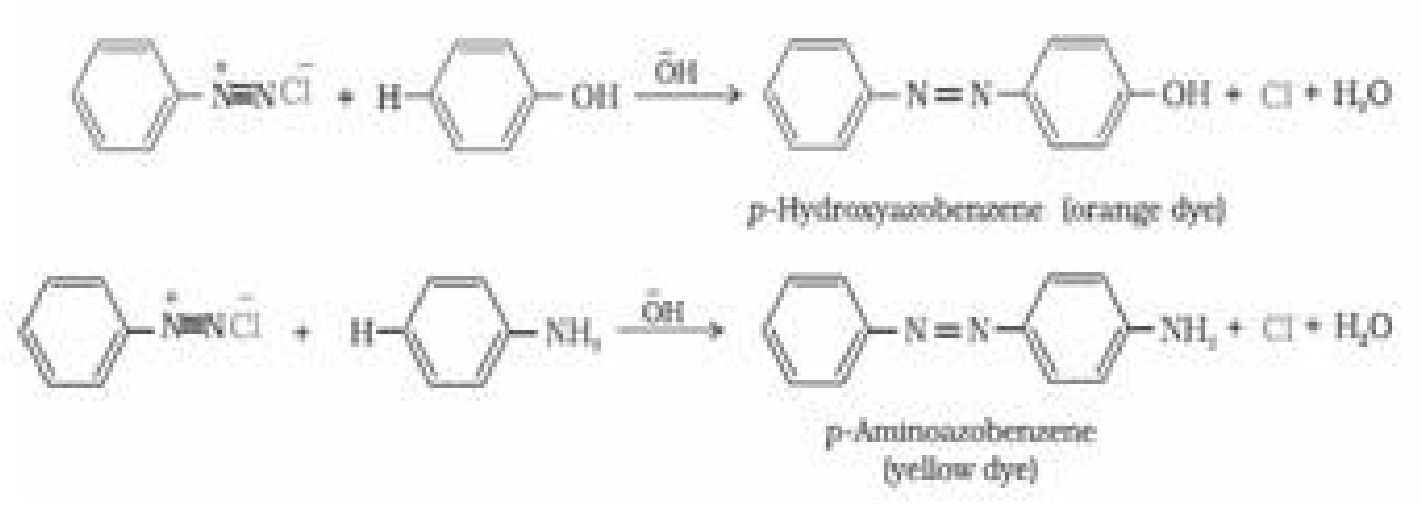
b) Reactions involving retention of diazo group, coupling reactions: Diazonium ion
acts as an electrophile because there is a positive charge on terminal nitrogen.
Therefore benzene diazonium chloride couples with electron rich compounds like
phenol and aniline to give azo compounds. Azo compounds contain -N=N- bond and
reaction is coupling reaction.